Figures & data
Figure 1 Scanning electron micrographs and size distribution plots of (A) copper, (B) palladium and (C) palladium@copper bimetallic nanoparticles.
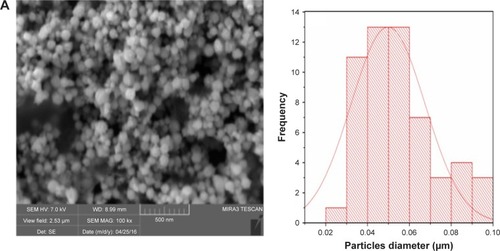
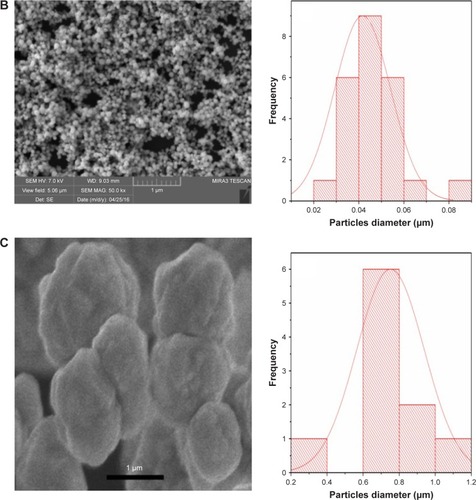
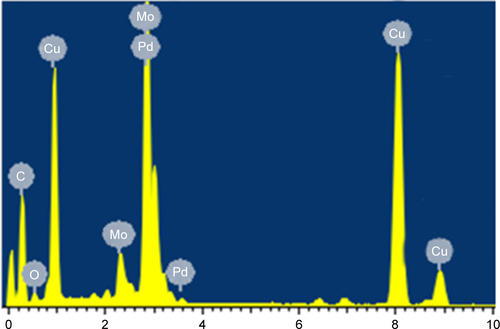
Figure 2 (A) X-ray diffraction pattern of palladium@copper bimetallic nanoparticles. (B) Energy-dispersive X-ray elemental mapping images of Pd, Cu, and Pd@Cu bimetallic nanoparticles, respectively.
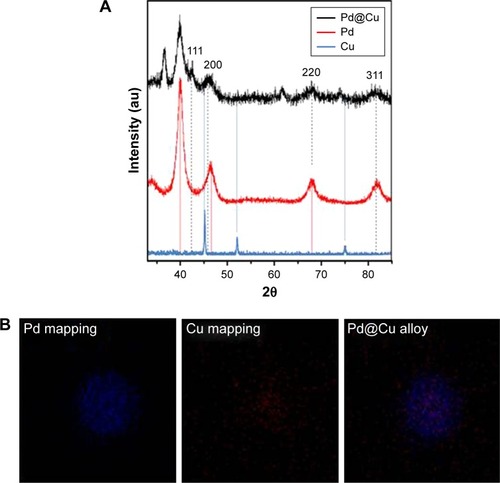
Figure 3 Absorbance spectra of (A) copper, (B) palladium and (C) palladium@copper bimetallic nanoparticles using different volumes of trisodium citrate (5 mM). The arrows show the maximum absorbance.
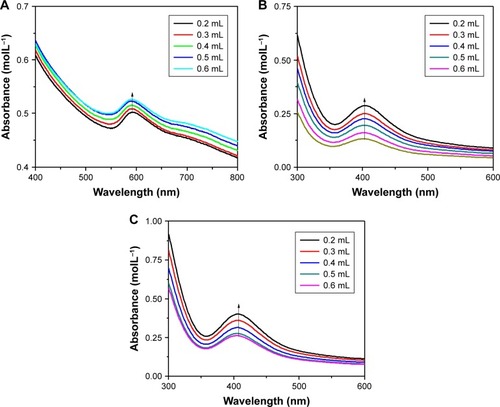
Figure 4 (A) Color change during catalytic reduction of 4-nitrophenol to 4-aminophenol and (B) ultraviolet–visible spectra of p-nitrophenol, p-nitrophenolate ion and 4-aminophenol. Reaction conditions: H2O (3 mL), Cu@Pd (15 μL, 0.1 mg mL−1), 4-nitrophenol (55 μL, 0.1 M) and NaBH4 (150 μL, 0.1 M). (a) 4-nitrophenol, (b) 4-nitrophenolate ion, (c) 4-aminophenol.
Abbreviations: NA, nitroaminophenol; NP, nitrophenol.
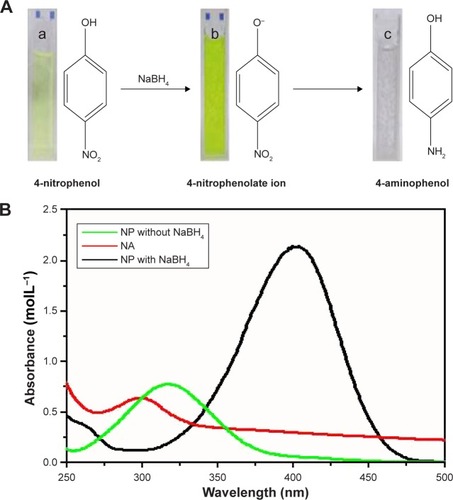
Figure 5 Ultraviolet–visible spectra for the reduction of 4-nitrophenol catalyzed by (A) copper, (B) palladium and (C) palladium@copper bimetallic nanoparticles.
Notes: Reaction conditions: H2O (3 mL), Cu (15 μL, 0.1 mg mL−1), 4-nitrophenol (40 μL, 0.1 M) and NaBH4 (150 μL, 0.1 M). (B) Conditions: H2O (3 mL), Pd (15 μL, 0.1 mg mL−1), 4-nitrophenol (40 μL, 0.1 M) and NaBH4 (150 μL, 0.1 M). (C) Conditions: H2O (3 mL), Pd@Cu (15 μL, 0.1 mg mL−1), 4-nitrophenol (40 μL, 0.1 M) and NaBH4 (150 μL, 0.1 M).
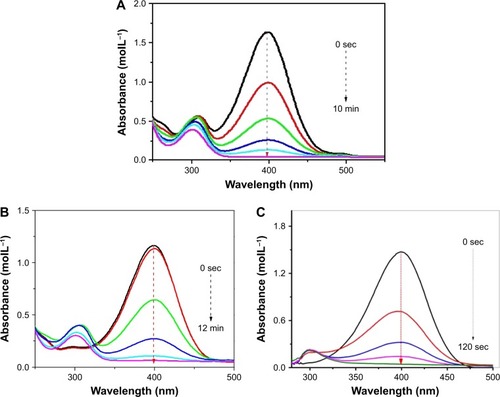
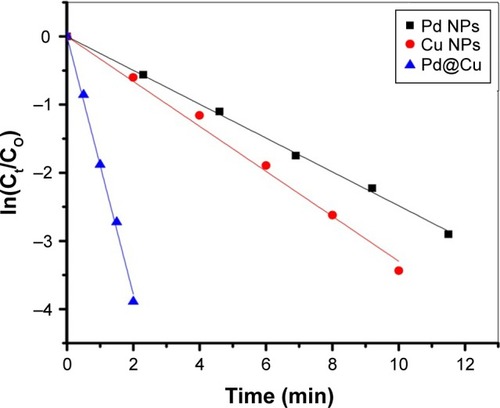
Figure 6 Determination of the reduction rates of 4-nitrophenol as a function of reaction time by using copper, palladium and palladium@copper bimetallic nanoparticles as a catalyst.
Notes: Reaction conditions: H2O (3 mL), Cu (15 μL, 0.1 mg mL−1), 4-nitrophenol (40 μL, 0.1 M) and NaBH4 (150 μL, 0.1 M). (b) Reaction conditions: H2O (3 mL), Pd (15 μL, 0.1 mg mL−1), 4-nitrophenol (40 μL, 0.1 M) and NaBH4 (150 μL, 0.1 M). (c) Reaction conditions: H2O (3 mL), Pd@Cu (15 μL, 0.1 mg mL−1), 4-nitrophenol (40 μL, 0.1 M) and NaBH4 (150 μL, 0.1 M).