Figures & data
Figure 1 The TEM, SE M, and AFM micrographs of the nanoparticles illustrate that the particles are properly dispersed, spherically-shaped, and have smooth surface characteristics. A, B, and C TEM images for the nanoparticles crosslinked with 40 μL of 5% (w/v) glutaraldehyde, 200 kV resolution. A) TEM image of the nanoparticles before ultrasonication which indicates that before ultrasonication the nanoparticles form clusters and are not properly dispersed. Bar, 500 nm. B) TEM image of the nanoparticles after ultrasonication for 15 minutes. Here it is demonstrated how ultrasonication assists in separating out the particles more uniformly and the particles are properly dispersed. Bar, 100 nm. C) TEM image of the nanoparticles after magnification. Bar, 100 nm. D) SEM image for the nanoparticles crosslinked with 40 μL of 5% (w/v) glutaraldehyde which demonstrates the smooth surface characteristics of the nanoparticles, 2 kV resolution. Bar, 1 μm. E) Three-dimensional AFM image of the nanoparticles. Bar, 300 nm.
Abbreviations: TEM, transmission electron microscopy; SEM, scanning electron microscopy; AFM, atomic force microscopy.
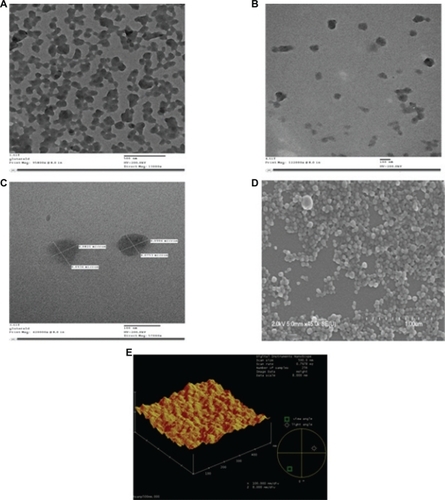
Figure 2 Zeta potential and particle sizer. A) The nanoparticles were prepared by adjusting the pH of the human serum albumin solution to 8.0 and controlling the amount of coacervation agent to form nanoparticles of size approximately 101.0 ± 0.9 nm. B) The protein nanoparticles were negatively charged and the zeta potential was −18 ± 2.9 mV at pH 7.0. The zeta potential of the glutaraldehyde crosslinked nanoparticles decreases when the concentration of glutaraldehyde is increased. Zeta potential measurements were performed in phosphate buffer at pH 7.0 because pH and ionic strength of the dilution medium affects the magnitude of the zeta potential. The polydispersity index was found to be 0.3.
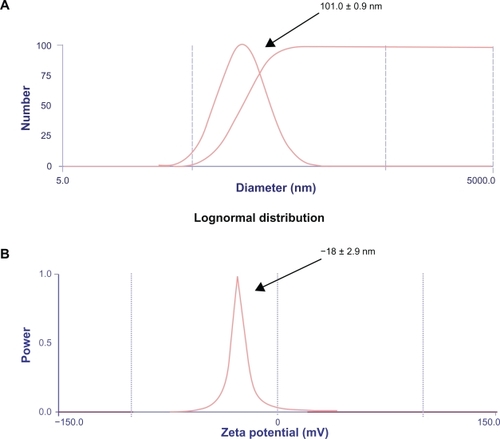
Figure 3 The cytotoxicity of the blank nanoparticles crosslinked with a series of glutaraldehyde concentrations was studied by incubating HUVECs for 96 hours with the nanoparticles. Percent cell viability over initial number of HUVECs treated with nanoparticles for up to 96 hours is shown. MTS assay was used to determine the cell viability after exposure to the nanoparticles. Nanoparticles without glutaraldehyde coating showed a cell viability of above 90% after 96 hours. Increasing the amount of glutaraldehyde in the particle preparation increased the cytotoxicity of the nanoparticles to the seeded HUVECs.
Abbreviation: HUVECs, human vascular enthothelial cells.
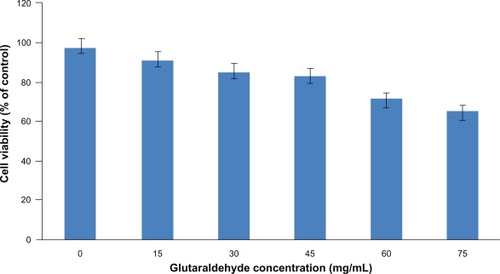
Figure 4 NPs sustain in-vitro protein release. A and B Indicate the cumulative amount of hAng-1 and hVEGF released from the NPs over a time period of two weeks. At the end of the two week incubation period it was observed that 49% of hAng-1 and 59% of hVEGF had been released from the NPs.
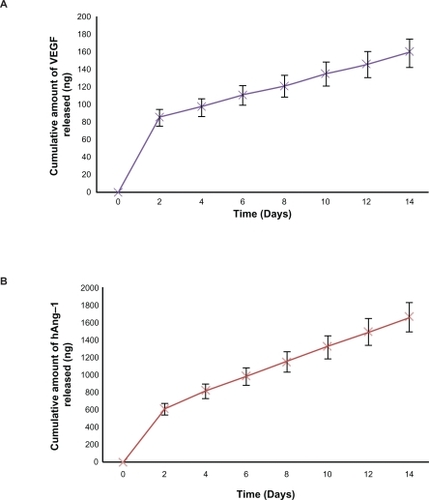
Figure 5 Bioactivity of human angiopoietin-1 and human VEGF loaded in nanoparticles. The proliferation of HUVECs in response to the nanoparticle supernatant was observed. HUVECS were seeded in a 96-well plate and the endothelial cell medium was replaced with nanoparticle supernatant with human VEGF antibody, nanoparticle supernatant with human angiopoietin-1 antibody, nanoparticle supernatant containing both human angiopoietin-1 and human VEGF, nanoparticle supernatant containing bovine serum albumin and endothelial cell medium (with 5% fetal bovine albumin) as the controls for 96 hours. The results were illustrated as the percent increase in cell proliferation relative to the unstimulated control. Least cell proliferation is observed with human angiopoietin-1 and maximum proliferation is observed in supernatant with a combination of both human angiopoietin-1 and human VEGF. The supernatant with bovine serum albumin showed a negligible effect as expected. An increasing cell proliferation is observed over the two-week incubation period.
Abbreviations: VEGF, vascular endothelial growth factor; HUVECs, human vascular endothelial cells.
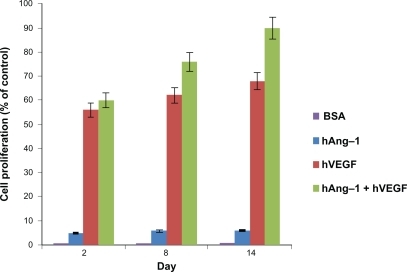
Figure 6 Human angiopoietin-1 and human VEGF were loaded into nanoparticles and the combined antiapoptotic effect of the proteins on the seeded HUVECs was demonstrated. HUVECs treated with serum-free control media without addition of human VEGF and human angiopoietin-1 proteins, always showed a percent apoptosis above 90%. The release kinetics shows that there is an increase in the cumulative amount of proteins released from the nanoparticles. The percent apoptosis due to nanoparticle supernatant containing human angiopoietin-1 (human VEGF antibody added) reduced from 31.5% to 27.06% for day 2 to day 14. Similarly, percent apoptosis due to nanoparticle supernatant containing human VEGF (human angiopoietin-1 antibody added) reduced from 29.6% to 27.74% for day 2 to day 14. The combined antiapoptotic effect of the nanoparticle supernatant containing human angiopoietin-1 and human VEGF decreased from 16.44% to 10.42% at day 2 to day 14. Thus, in comparison with the control, cell apoptosis decreased dramatically (almost by 50%) with the application of supernatant from nanoparticles loaded with both the proteins.
Abbreviations: VEGF, vascular endothelial growth factor; HUVECs, human vascular endothelial cells.
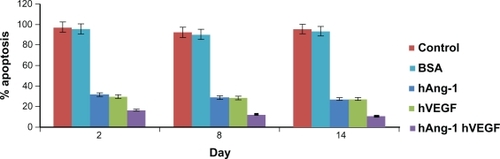
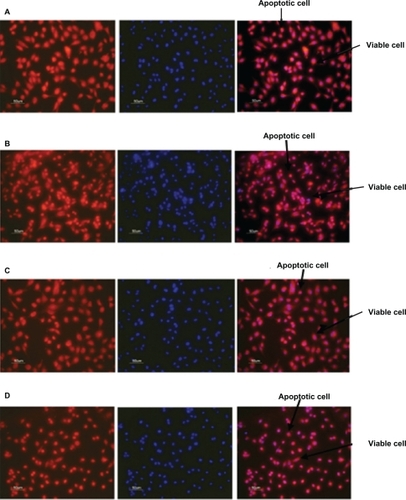
Figure 7 Fluorescent microscope images showing antiapoptotic effect of human angiopoietin-1 and human VEGF on HUVECs at day 14. Bar, 50 μm. It was observed that on day 14, the proteins coencapsulated in the nanoparticles significantly decreased HUVEC apoptosis compared with the control, as well as the individual proteins. Human vascular endothelial cells were seeded onto a 96-well plate at a cell density of 2 × 104 cells per well. The medium was then replaced with 0.1 mL of A) nanoparticle supernatant, B) nanoparticle supernatant with excess human VEGF antibody, C) nanoparticle supernatant with excess human angiopoietin-1 antibody and incubated for 96 hours. D) Control cultures received the same amount of serum-free media without addition of human VEGF and human angiopoietin-1 proteins. The numbers of cells floating in each well were collected after phosphate-buffered saline washing and counted. The number of apoptotic cells in the adherent cells was then determined after 96 hours using the MitoTracker® Red CMXRos kit and DAPI nucleic acid staining and counted using fluorescence microscopy.
Abbreviations: HUVECs, human vascular endothelial cells; VEGF vascular endothelial growth factor.