Figures & data
Table 1 Primers for quantitative real-time PCR analysis
Figure 1 Effects of hBD3 on the cell viability and osteogenic differentiation of hPDLCs in inflammatory microenvironments.
Notes: hPDLCs were treated with hBD3 (5 μg/mL) and E. coli-LPS (1 μg/mL). (A) The cell viability of hPDLCs was analyzed with a CCK-8 assay on day 7. (B) ALP staining and (C) ALP activity of hPDLCs after hBD3 treatment. (D) ALP, COL-1, and Runx-2 mRNA expressions on day 7 analyzed by real-time PCR. #P<0.05 and ##P<0.01 compared with the control group; *P<0.05, **P<0.01, and ****P<0.0001.
Abbreviations: ALP, alkaline phosphatase; CCK-8, cell counting kit-8; COL-1, collagenase-I; E. coli, Escherichia coli; hBD3, human β-defensin 3; hPDLCs, human periodontal ligament cells; LPS, lipopolysaccharides; PCR, polymerase chain reaction; Runx-2, runt-related transcription factor 2.
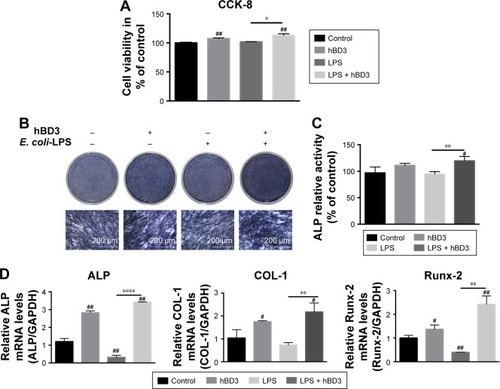
Figure 2 Effects of hBD3-combined AuNPs on cell viability of hPDLCs and cellular uptake of AuNPs in inflammatory microenvironments.
Notes: hPDLCs were treated with hBD3 (5 μg/mL), AuNPs (45 nm, 10 μM), and E. coli-LPS (1 μg/mL). (A) The biocompatibility of hPDLCs measured by CCK-8 on day 7. (B) TEM images of uptake of AuNPs on day 7. Arrows indicate internalized AuNPs. #P<0.05 and ##P<0.01 compared with the control group.
Abbreviations: AuNPs, gold nanoparticles; CCK-8, cell counting kit-8; E. coli, Escherichia coli; hBD3, human β-defensin 3; hPDLCs, human periodontal ligament cells; LPS, lipopolysaccharides; TEM, transmission electron microscopy.
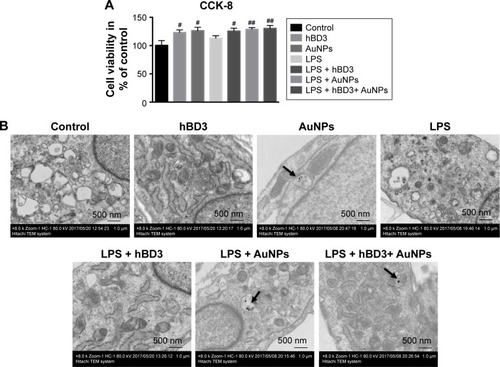
Figure 3 Effects of hBD3-combined AuNPs on the ALP activity and calcium deposition of hPDLCs in inflammatory microenvironments.
Notes: hPDLCs were treated with hBD3 (5 μg/mL), AuNPs (45 nm, 10 μM), and E. coli-LPS (1 μg/mL). (A) ALP staining on day 7 and calcium deposition staining with ARS on day 21. (B) ALP activity levels on day 7 and (C) calcium deposition quantification on day 21. #P<0.05, ##P<0.01, ###P<0.001, and ####P<0.0001 compared with the group control; *P<0.05 and ****P<0.0001.
Abbreviations: ALP, alkaline phosphatase; ARS, alizarin red S; AuNPs, gold nanoparticles; E. coli, Escherichia coli; hBD3, human β-defensin 3; hPDLCs, human periodontal ligament cells; LPS, lipopolysaccharides.
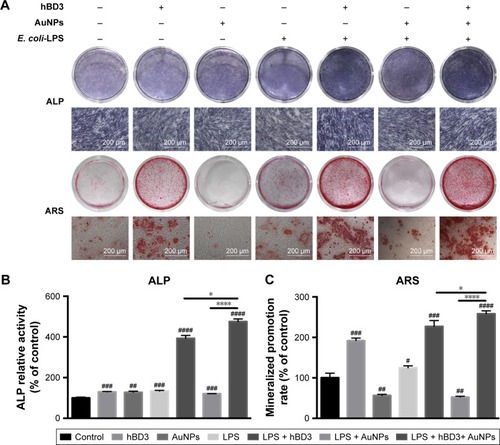
Figure 4 Effects of hBD3-combined AuNPs on osteogenic gene and protein expressions of hPDLCs in inflammatory microenvironments.
Notes: hPDLCs were treated with hBD3 (5 μg/mL), AuNPs (45 nm, 10 μM), and E. coli-LPS (1 μg/mL). (A) The mRNA expression levels of ALP, COL-1, and Runx-2 analyzed by real-time PCR on day 7. (B) The protein expression levels of ALP, COL-1, and Runx-2 analyzed by Western blot on day 7. #P<0.05, ##P<0.01, ###P<0.001, and ####P<0.0001 compared with the control group; *P<0.05, **P<0.01, ***P<0.001, and ****P<0.0001.
Abbreviations: ALP, alkaline phosphatase; AuNPs, gold nanoparticles; COL-1, collagenase-I; E. coli, Escherichia coli; hBD3, human β-defensin 3; hPDLCs, human periodontal ligament cells; LPS, lipopolysaccharides; PCR, polymerase chain reaction; Runx-2, runt-related transcription factor 2.
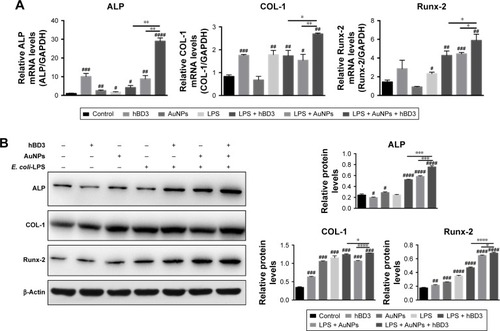
Figure 5 Effects of Wnt/β-catenin signaling pathway on osteogenic differentiation of hPDLCs induced by hBD3-combined AuNPs in inflammatory microenvironments.
Notes: hPDLCs were treated with hBD3 (5 μg/mL), AuNPs (45 nm, 10 μM), E. coli-LPS (1 μg/mL) and ICG-001 (10 μM). (A) The Wnt/β-catenin pathway target gene cyclin D1 mRNA expression on day 7 analyzed by real-time PCR. (B) β-Catenin and cyclin D1 protein expressions on day 7 analyzed by Western blot. (C) ICG-001 blocked the mRNA expression of the target gene cyclin D1 and (D) the protein expressions of β-catenin and cyclin on day 7. #P<0.05, ##P<0.01, ###P<0.001, and ####P<0.0001 compared with the control group; *P<0.05, **P<0.01, ***P<0.001, and ****P<0.0001.
Abbreviations: AuNPs, gold nanoparticles; E. coli, Escherichia coli; hBD3, human β-defensin 3; hPDLCs, human periodontal ligament cells; LPS, lipopolysaccharides; PCR, polymerase chain reaction.
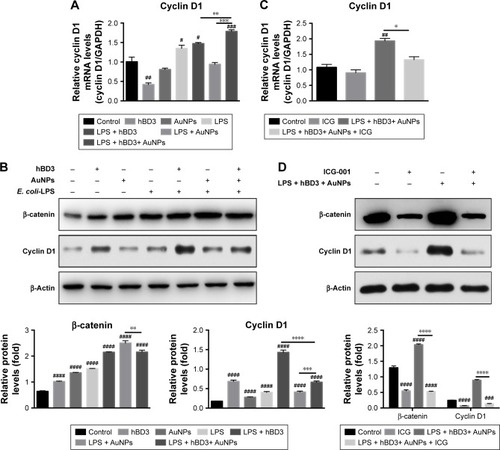
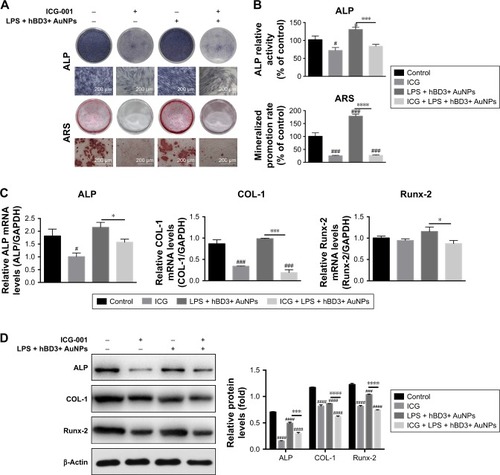
Figure 6 Effects of ICG-001 on osteogenic differentiation of hPDLCs induced by hBD3-combined AuNPs in inflammatory microenvironments.
Notes: hPDLCs were treated with hBD3 (5 μg/mL), AuNPs (45 nm, 10 μM), E. coli-LPS (1 μg/mL) and ICG-001 (10 μM). (A) ALP staining on day 7 and mineralized nodules staining with ARS on day 21 and (B) ALP activity levels on day 7 and mineralized nodules activity levels on day 21. (C) ALP, COL-1, and Runx-2 mRNA expressions on day 7 analyzed by real-time PCR and (D) ALP, COL-1, and Runx-2 protein expressions on day 7 analyzed by Western blot. #P<0.05, ###P<0.001, and ####P<0.0001 compared with the control group; *P<0.05, ***P<0.001, and ****P<0.0001.
Abbreviations: ALP, alkaline phosphatase; ARS, alizarin red S; AuNPs, gold nanoparticles; COL-1, collagenase-I; E. coli, Escherichia coli; hBD3, human β-defensin 3; hPDLCs, human periodontal ligament cells; PCR, polymerase chain reaction; Runx-2, runt-related transcription factor 2.