Figures & data
Figure 1 The temperature sensibility of PNIPAM and PM.
Notes: (A) Different solution states of P(NIPAM-co-Am) at room temperature (a) and 42°C (b). (B) The LCST detected spectrophotometrically with the solution being heated at a speed of 1°C/min. The temperature of the initial polymer solution with 90% of initial transmittance (at 460 nm) was defined as the LCST. The red curve is the transmittance curve of PNIPAM and the black curve is the transmittance curve of PM.
Abbreviations: Am, acrylamide; LCST, lower critical solution temperature; PM, copolymer of N-isopropylacrylamide and acrylamide; PNIPAM, poly(N-isopropylacrylamide).
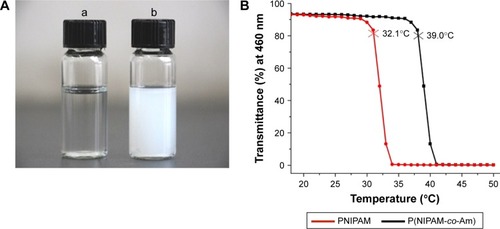
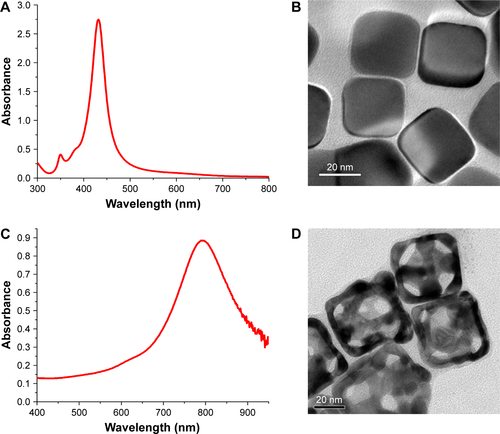
Figure 2 Characterization of AuNCs-PM and DOX/AuNCs-PM.
Notes: (A) TEM images of the AuNCs-PM. A magnified TEM picture of the AuNCs-PM is shown in the inset. (B) UV–Vis absorption spectra of AuNCs and AuNCs-PM. (C) Mean hydrodynamic diameters of AuNCs and AuNCs-PM plotted as a solution temperature function. (D) UV–Vis absorption spectra of AuNCs, DOX solution, AuNCs-PM and DOX/AuNCs-PM.
Abbreviations: AuNCs, gold nanocages; AuNCs-PM, PM-grafted AuNCs; DOX, doxorubicin; DOX/AuNCs-PM, DOX-loaded and PM-grafted AuNCs; DOX/AuNCs-PM-HA, DOX-loaded, PM-grafted and HA-modified AuNCs; HA, hyaluronic acid; PM, copolymer of N-isopropylacrylamide and acrylamide; TEM, transmission electron microscopy; UV–Vis, ultraviolet–visible.
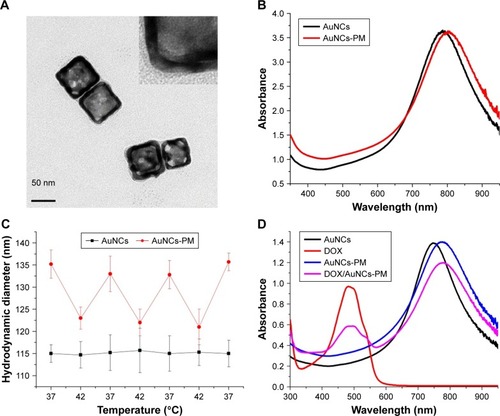
Figure 3 Characterization, photothermal conversion and the release profile of functionalized AuNCs.
Notes: (A) Sizes of AuNCs, AuNCs-PM, DOX/AuNCs-PM and DOX/AuNCs-PM-HA. (B) Zeta potentials of AuNCs, AuNCs-PM, DOX/AuNCs-PM and DOX/AuNCs-PM-HA. (C) Photothermal curves of the AuNCs-PM-HA (AuNCs content at 40 μg/mL) and H2O irradiated by NIR laser (808 nm, 1.5 W/cm2). (D and E) DOX release curves of DOX/AuNCs-PM-HA at pH 7.4 and pH 4.5, with or without HAase. (F) DOX release curves of DOX/AuNCs-PM-HA at pH 7.4 and pH 4.5 with NIR laser irradiation (808 nm, 1.5 W/cm2, 7 min), with or without HAase.
Abbreviations: AuNCs, gold nanocages; AuNCs-PM, PM-grafted AuNCs; DOX, doxorubicin; DOX/AuNCs-PM, DOX-loaded and PM-grafted AuNCs; DOX/AuNCs-PM-HA, DOX-loaded, PM-grafted and HA-modified AuNCs; HA, hyaluronic acid; HAase, hyaluronidase; NIR, near-infrared irradiation; PM, copolymer of N-isopropylacrylamide and acrylamide.
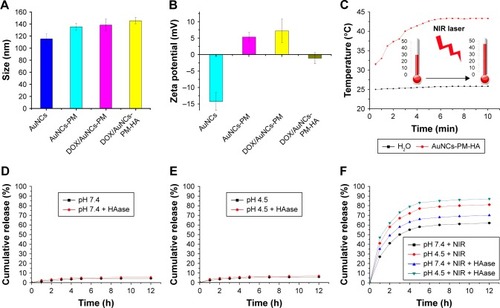
Figure 4 Cellular uptake of SMMC-7721 cells. SMMC-7721 cells stained by DAPI and cultured with DOX/AuNCs-PM and DOX/AuNCs-PM-HA (DOX and AuNCs content at 1.68 and 40 μg/mL, respectively) for 4 h at 37°C; magnification ×400.
Notes: (A) DOX/AuNCs-PM, (B) DOX/AuNCs-PM-HA, (C) DOX/AuNCs-PM-HA+NIR (808 nm, 1.5 W/cm2, 7 min). Blue fluorescence: nuclei stained by DAPI, red fluorescence: DOX in cells.
Abbreviations: AuNCs, gold nanocages; DAPI, 4,6-diamidimo-2-phenylindole; DOX, doxorubicin; DOX/AuNCs-PM, DOX-loaded and PM-grafted AuNCs; DOX/AuNCs-PM-HA, DOX-loaded, PM-grafted and HA-modified AuNCs; DOX/AuNCs-PM-HA+NIR, DOX/AuNCs-PM-HA with NIR laser; HA, hyaluronic acid; NIR, near-infrared irradiation; PM, copolymer of N-isopropylacrylamide and acrylamide.
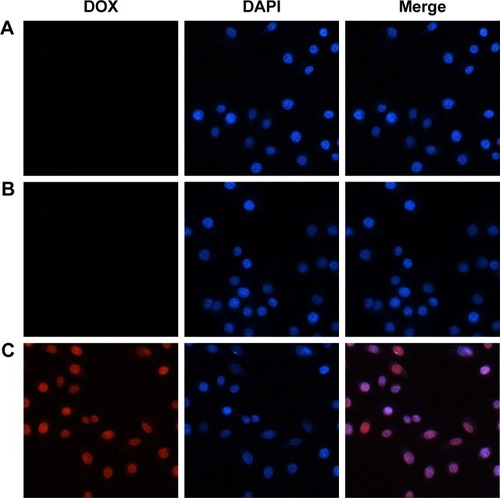
Figure 5 Cell viability of SMMC-7721 cells.
Notes: (A) Cell viability of SMMC-7721 cells incubated for 24 h with different concentrations of AuNCs-PM-HA with or without NIR irradiation (808 nm, 1.5 W/cm2, 7 min). (B) Viability of SMMC-7721 cells incubated for 24 h with DOX/AuNCs-PM-HA with or without NIR, free DOX (DOX content at 0, 0.42, 0.84, 1.26, 1.68 μg/mL) and AuNCs-PM-HA with NIR irradiation (808 nm, 1.5 W/cm2, 7 min). *p<0.05, **p<0.01, and ***p<0.001 indicate significant difference between two groups.
Abbreviations: AuNCs, gold nanocages; DOX, doxorubicin; DOX/AuNCs-PM-HA, DOX-loaded, PM-grafted and HA-modified AuNCs; HA, hyaluronic acid; NIR, near-infrared irradiation; PM, copolymer of N-isopropylacrylamide and acrylamide.
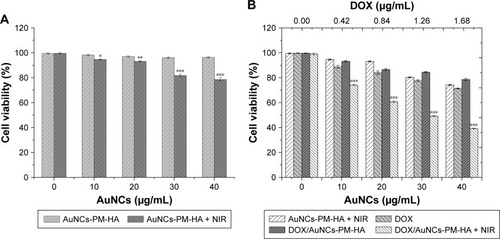
Figure 6 Fluorescence microscopy images of DOX in slices of tumors and other organs after injection (0.5 and 6 h) with normal saline (control), DOX solution (free DOX), DOX/AuNCs-PM-HA (200×).
Notes: The scale bars represent 500 μm. The red fluorescence is expressed by released DOX. The tumors of DOX/AuNCs-PM-HA group were illuminated by NIR laser (1.5 W/cm2, 808 nm, 7 min) at 0.5 and 6 h postinjection, respectively.
Abbreviations: AuNCs, gold nanocages; DOX, doxorubicin; DOX/AuNCs-PM-HA, DOX-loaded, PM-grafted and HA-modified AuNCs; HA, hyaluronic acid; NIR, near-infrared irradiation; PM, copolymer of N-isopropylacrylamide and acrylamide.
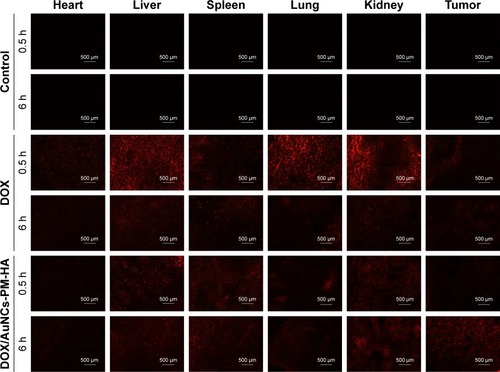
Figure 7 In vivo photoacoustic tomography image of H22 tumor (marked as green)-bearing mice treated with normal saline, AuNCs, DOX/AuNCs-PM-HA (AuNCs content at 23.78 mg/kg).
Note: The scale bars represent 3 mm. The tumor areas are marked by red ellipses.
Abbreviations: AuNCs, gold nanocages; DOX, doxorubicin; DOX/AuNCs-PM-HA, DOX-loaded, PM-grafted and HA-modified AuNCs; H22, hepatocarcinoma 22; HA, hyaluronic acid; PM, copolymer of N-isopropylacrylamide and acrylamide.
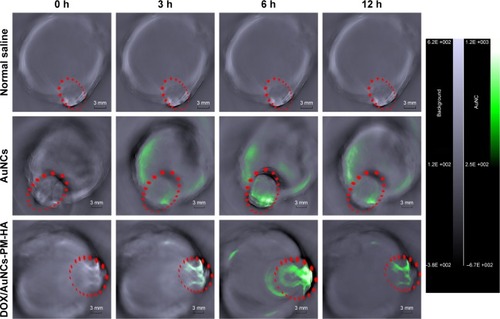
Figure 8 In vivo treatment on H22 tumor-bearing mice with the DOX/AuNCs-PM-HA.
Notes: (A) Body weight changes with times of H22 tumor-bearing mice after first treatment with different formulations (n=5, mean ± SD). (B) Hematoxylin and eosin staining of tumor sections collected from different treatment groups (normal saline [control], DOX solution, DOX/AuNCs-PM-HA, DOX/AuNCs-PM-HA+NIR [1.5 W/cm2, 808 nm, 7 min] [DOX content at 2 mg/kg]). The scale bars represent 500 μm.
Abbreviations: AuNCs, gold nanocages; DOX, doxorubicin; DOX/AuNCs-PM-HA, DOX-loaded, PM-grafted and HA-modified AuNCs; DOX/AuNCs-PM-HA+NIR, DOX/AuNCs-PM-HA with NIR laser; H22, hepatocarcinoma 22; HA, hyaluronic acid; NIR, near-infrared irradiation; PM, copolymer of N-isopropylacrylamide and acrylamide.
![Figure 8 In vivo treatment on H22 tumor-bearing mice with the DOX/AuNCs-PM-HA.Notes: (A) Body weight changes with times of H22 tumor-bearing mice after first treatment with different formulations (n=5, mean ± SD). (B) Hematoxylin and eosin staining of tumor sections collected from different treatment groups (normal saline [control], DOX solution, DOX/AuNCs-PM-HA, DOX/AuNCs-PM-HA+NIR [1.5 W/cm2, 808 nm, 7 min] [DOX content at 2 mg/kg]). The scale bars represent 500 μm.Abbreviations: AuNCs, gold nanocages; DOX, doxorubicin; DOX/AuNCs-PM-HA, DOX-loaded, PM-grafted and HA-modified AuNCs; DOX/AuNCs-PM-HA+NIR, DOX/AuNCs-PM-HA with NIR laser; H22, hepatocarcinoma 22; HA, hyaluronic acid; NIR, near-infrared irradiation; PM, copolymer of N-isopropylacrylamide and acrylamide.](/cms/asset/854c82fb-1611-43f7-ab08-4b4b128ce25e/dijn_a_12193896_f0008_c.jpg)
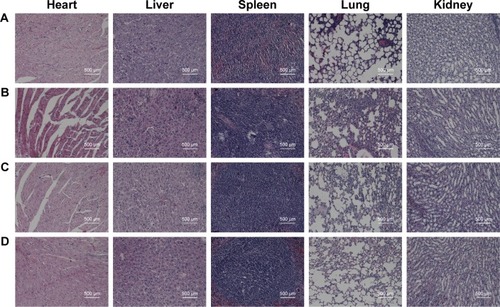
Figure 9 Histological observation of major organs (hearts, livers, spleens, lungs and kidneys) and tumor sections. The organs were collected from different groups after treatment with different formulations and were stained by H&E.
Notes: (A) Normal saline (control), (B) DOX solution, (C) DOX/AuNCs-PM-HA and (D) DOX/AuNCs-PM-HA+NIR (1.5 W/cm2, 808 nm, 7 min) (DOX content at 2 mg/kg). The scale bars represent 500 μm.
Abbreviations: AuNCs, gold nanocages; DOX, doxorubicin; DOX/AuNCs-PM-HA, DOX-loaded, PM-grafted and HA-modified AuNCs; DOX/AuNCs-PM-HA+NIR, DOX/AuNCs-PM-HA with NIR laser; HA, hyaluronic acid; NIR, near-infrared irradiation; PM, copolymer of N-isopropylacrylamide and acrylamide.
Scheme 1 Schematic illustration for the preparation of the tumor-targeted, multi-stimuli responsive drug delivery system (DOX/AuNCs-PM-HA) as a combination of photoacoustic tomography and photothermal-chemotherapy.
Note: **p<0.01 and ***p<0.001 represent significant difference between two groups.
Abbreviations: DOX, doxorubicin; AuNCs, gold nanocages; P(NIPAM-co-Am), copolymer of N-isopropylacrylamide and acrylamide; PM, copolymer of N-isopropylacrylamide and acrylamide; HA, hyaluronic acid; DOX/AuNCs-PM-HA, DOX-loaded, PM-grafted and HA-modified AuNCs; IV, intravenous; NIR, near-infrared irradiation; photothermal-chemotherapy, combination of photothermal therapy and chemotherapy.
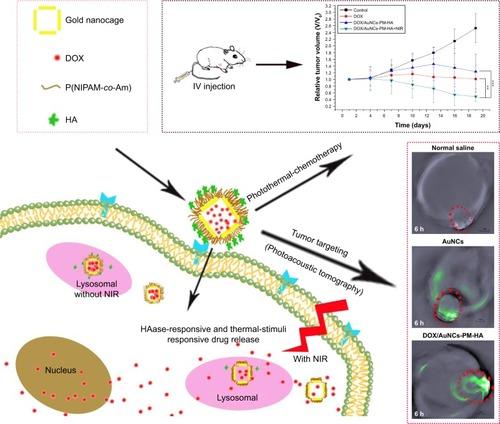
Figure S1 Synthesis of P(NIPAM-co-Am)-SH copolymers through RAFT copolymerization and end group modification with n-butylamine (nBuNH2).
Abbreviations: AIBN, 2,2′-azobis(isobutyronitrile); Am, acrylamide; DIMA, 2-(dodecylthiocarbonothioylthio)-2-methylpropionic acid N-hydroxysuccinimide ester; NIPAM, N-isopropylacrylamide; RAFT, reversible addition-fragmentation chain transfer; SH, sulfydryl; TCA, trithiocarbonate.
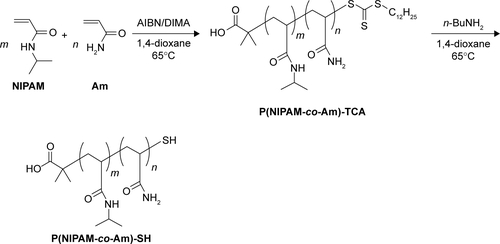
Figure S2 1H-NMR spectrum and structure of P(NIPAM-co-Am) copolymers (in CDCl3). 1.02 ppm (d, −CH2CH(CH3)2), 1.16 ppm (s, −NHCH(CH3)2), 1.30–2.40 ppm (m, polymer backbone protons), 4.03 ppm (s, −NHCH), 4.22 ppm (s, −C(=O)OCH2), 6.46 ppm (bs, −C(=O)NH).
Abbreviations: Am, acrylamide; bs, broad singlet proton; d, doublet proton; m, multiplet proton; NIPAM, N-isopropylacrylamide; NMR, nuclear magnetic resonance; s, singlet proton.
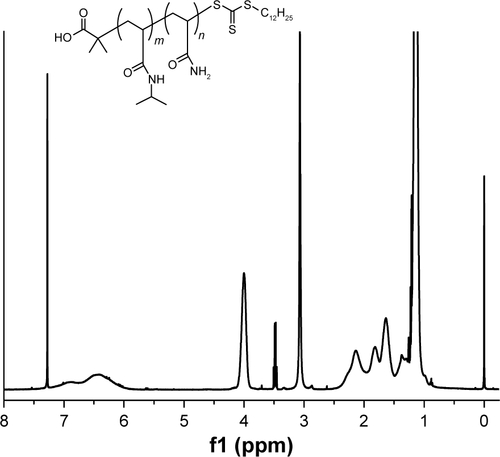
Figure S3 (A) Kinetic plot of ln(M)0/(M) vs time and (B) the plot of number-average molecular weights (Mn) vs monomer conversion for polymerization of NIPAM and Am mediated by DIMA and initiated by AIBN at 65°C in 1,4-dioxane.
Abbreviations: AIBN, 2,2′-azobis(isobutyronitrile); Am, acrylamide; DIMA, 2-(dodecylthiocarbonothioylthio)-2-methylpropionic acid N-hydroxysuccinimide ester; NIPAM, N-isopropylacrylamide.
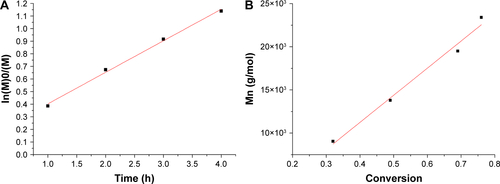
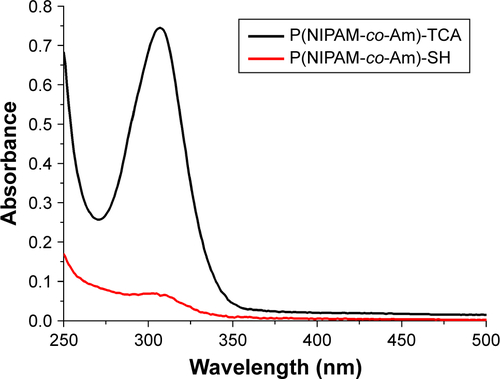
Figure S4 UV–Vis spectra of poly(NIPAM-co-Am) copolymers (PM, black line) and the aminolysis polymer P(NIPAM-co-Am)-SH (PM-SH, red line) at the concentration of 1 mg/mL in ethanol. The aminolysis of poly(NIPAM-co-Am) copolymer was conducted in the presence of butylamine in 1,4-dioxane at 25°C.
Abbreviations: Am, acrylamide; NIPAM, N-isopropylacrylamide; PM, copolymer of N-isopropylacrylamide and acrylamide; SH, sulfydryl; UV, ultraviolet; Vis, visible.