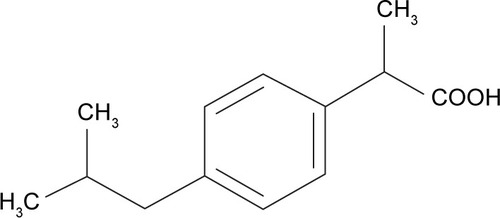
Figures & data
Figure 2 Graphical abstract showing nanocrystals formation and their interaction with polymers.
Note: ***Outstanding significant.
Abbreviation: MD, molecular dynamics.
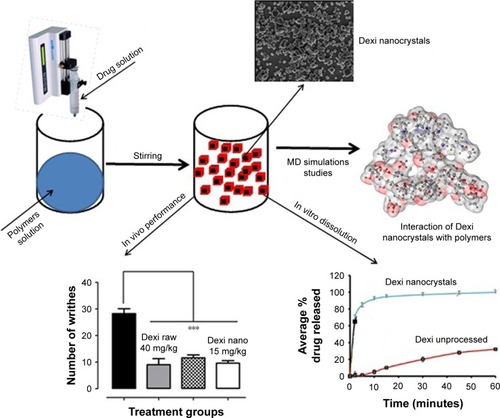
Table 1 Particle sizes and PDI values for Dexi nanocrystals produced using different polymer combinations
Figure 3 Particle size measurements of Dexi–HPMC–polyvinyl pyrrolidone (A) and Dexi–HPMC–Eudragit (B) nanocrystals.
Abbreviations: Dexi, dexibuprofen; HPMC, hydroxypropyl methyl cellulose.
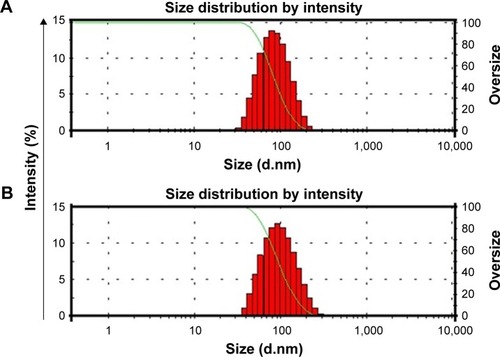
Figure 4 Scanning electron microscopy micrographs for unprocessed Dexi (A) and transmission electron microscopy images of Dexi nanocrystals (B).
Abbreviation: Dexi, dexibuprofen.
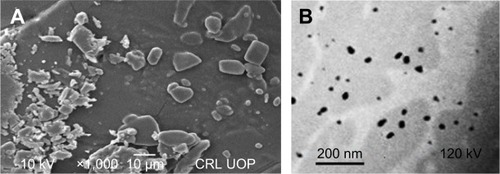
Figure 5 Differential scanning calorimetry thermograms of polyvinyl pyrrolidone K-30 (A), unprocessed Dexi (B), Dexi nanocrystals (C), Eudragit RS100 (D), and hydroxypropyl methyl cellulose (E).
Abbreviation: Dexi, dexibuprofen.
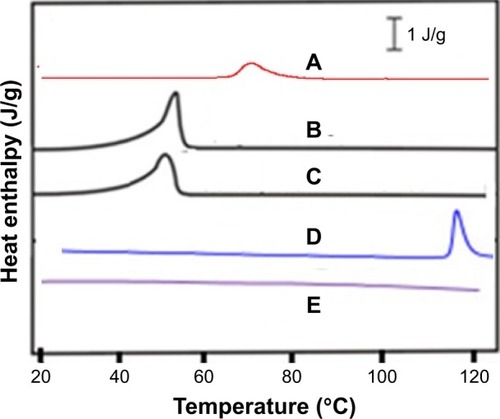
Figure 6 Powder X-ray diffraction diffractograms of Dexi nanocrystals, unprocessed Dexi, and chosen polymers.
Abbreviations: Dexi, dexibuprofen; EUD, Eudragit; HPMC, hydroxypropyl methyl cellulose.
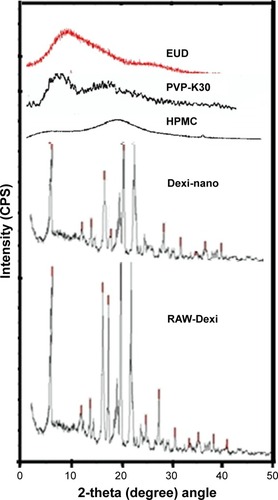
Figure 7 Fourier transform infrared spectra of hydroxypropyl methyl cellulose (A), unprocessed Dexi (B), Dexi nanocrystals (C), polyvinyl pyrrolidone K-30 (D), and Eudragit RS100 (E).
Abbreviation: Dexi, dexibuprofen.
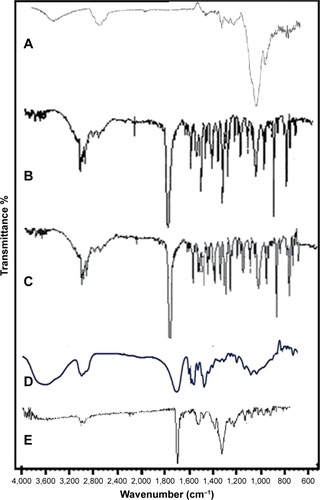
Figure 8 Solubility studies of Dexi nanocrystals, unprocessed Dexi in pure water and stabilizer solutions.
Abbreviations: Dexi, dexibuprofen; EUD, Eudragit; HPMC, hydroxypropyl methyl cellulose; PVP, polyvinyl pyrrolidone.
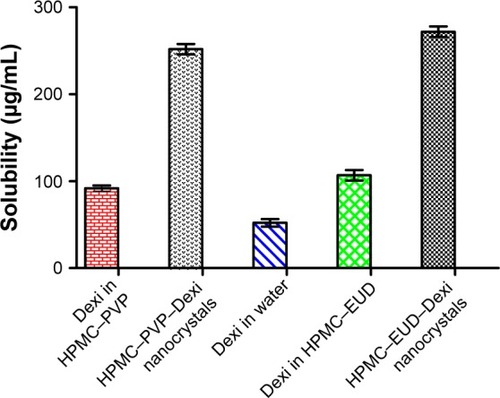
Table 2 The binding energies for Dexi–polymer complexes obtained from docking calculations
Figure 9 Interactions of HPMC–Dexi (top) and HPMC–PVP–Dexi (bottom) complexes showing hydrogen bonding as black lines.
Note: (A) Hydrophobic interactions between the drug and the PVP side chains may have contributed to the enhanced binding, (B) molecular surface of the polymers is displayed according to polarity.
Abbreviations: Dexi, dexibuprofen; HPMC, hydroxypropyl methyl cellulose; PVP, polyvinyl pyrrolidone.
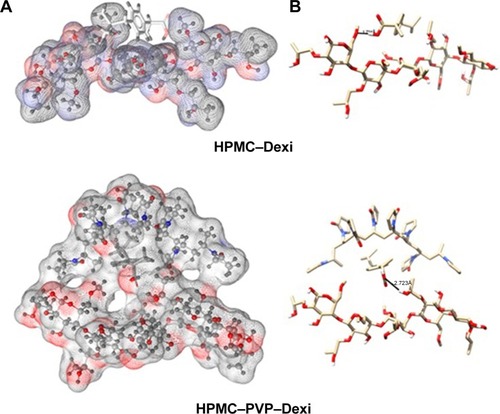
Figure 10 Interactions of EUD–Dexi (top) and HPMC–EUD–Dexi (bottom) complexes showing hydrogen bonding as black lines.
Note: (A) Enhanced binding might be due to hydrogen bonding formation with both polymeric units, (B) molecular surface of the polymers is displayed according to polarity.
Abbreviations: Dexi, dexibuprofen; EUD, Eudragit; HPMC, hydroxypropyl methyl cellulose.
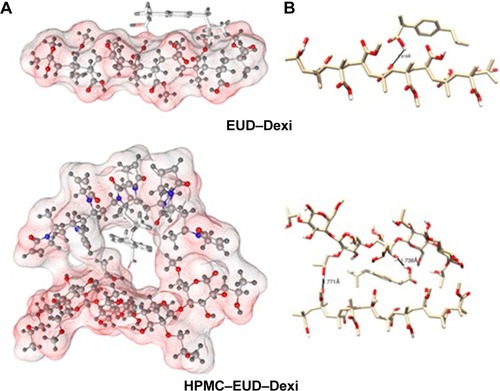
Figure 11 Comparative dissolution studies of Dexi nanocrystals and its counterparts including tablets, microsuspension, and raw Dexi.
Abbreviations: Dexi, dexibuprofen; EUD, Eudragit; HPMC, hydroxypropyl methyl cellulose.
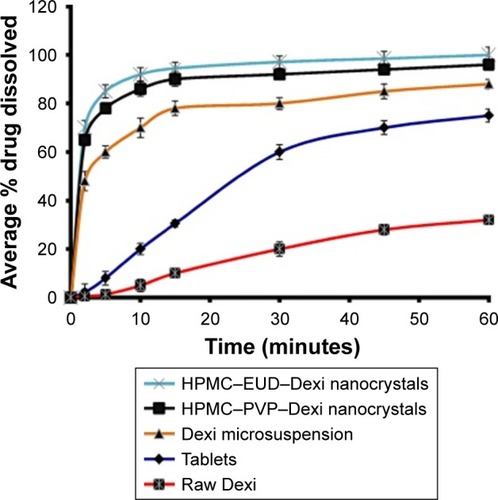
Table 3 Monitoring of particle size measurements of Dexi nanocrystals for 90 days at 25°C
Figure 12 Comparative physical stability studies of Dexi nanocrystals as a function of time while monitoring the particle sizes and PDI values at 2°C–8°C (A), 25°C (B), and 40°C (C).
Abbreviation: Dexi, dexibuprofen.
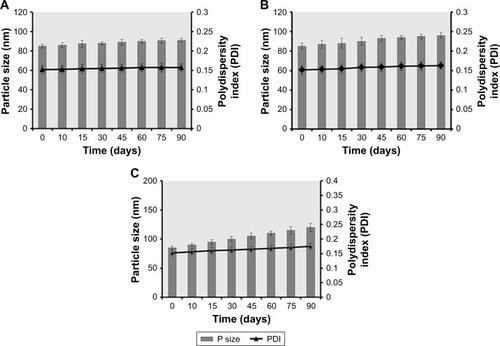
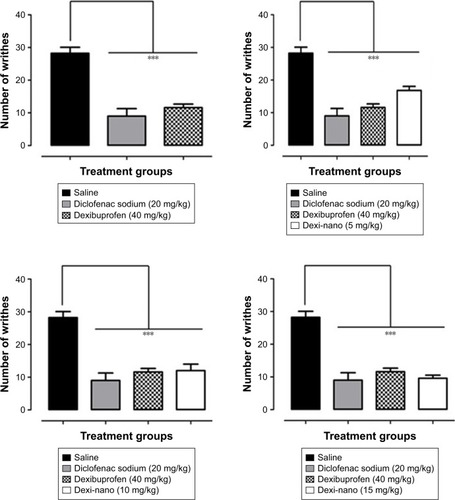
Figure 13 Comparative antinociceptive effect of Dexi nanocrystals and unprocessed counterpart at graded doses of 5–15 mg/kg in the acetic acid-induced abdominal constriction assay.
Notes: Data presented as mean ± standard error of the mean. ***P<0.001 as compared to saline-treated group. No significant difference was observed between Dexi (40 mg/kg)-treated group and groups treated with Dexi-nano (5, 10, and 15 mg/kg). One-way analysis of variance followed by Tukey’s post hoc multiple comparison test. n=6 mice per group.
Abbreviation: Dexi, dexibuprofen.