Figures & data
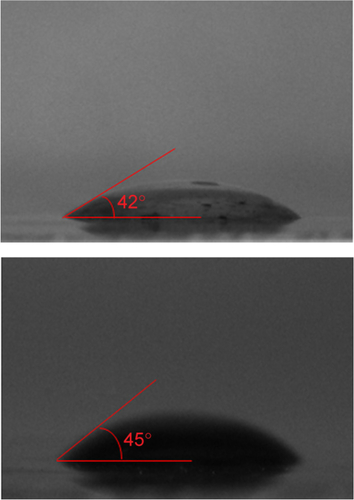
Figure 1 Water solubility, wetting transparency, and surface energy of GQDs.
Notes: (A) The absorbance (λex =275 nm) as a function of concentration, where A and l represent absorbency and cell length respectively. The experimental data (symbols) are well described by the Lambert-Beer law (line), which indicates good water solubility of the prepared GQDs. (B) UV/Vis absorption spectra of GQD having concentrations of 150, 125, 100, 75, 50, 25, 12.5, and 6 µg/mL indicate band around 260 nm. (C) Photograph of a 10 µL drop of water on the GQDs, showing a water contact angle of 14°. (D) Photograph of a 10 µL drop of diiodomethane on the GQDs with a contact angle of 46°.
Abbreviation: GQDs, graphene quantum dots.
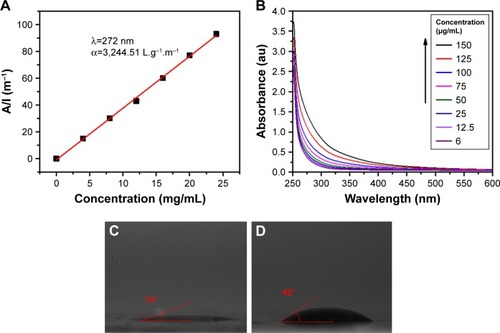
Figure 2 Fluorescence intensity of trypsin, substrate, and trypsin+substrate as a function of time and substrate concentration.
Notes: Fluorogenic substrate, Boc-Gln-Ala-Arg-AMC, at different concentrations (0, 0.1, 0.25, 0.5, and 1 µm) was incubated with 1% trypsin in 96-well plates at different time points (2, 10, 30, and 60 min). (A–D) Different concentrations of substrate over different time points compared to only trypsin and substrate. (E) Highest concentration of substrate compared to substrate and trypsin only. Fluorescence signals were measured using plate reader at Ex/Em: 355/450 nm, where Ex and Em represents excitation and emission wavelengths. Control wells contained H2O+substrate and H2O+trypsin.
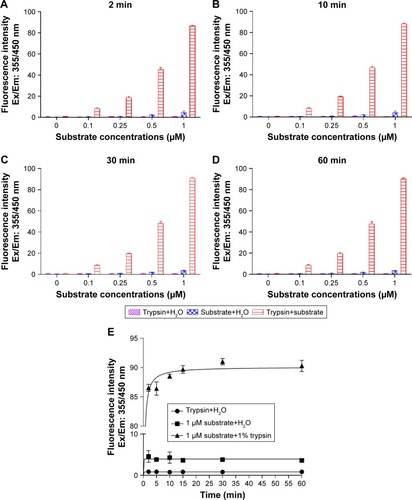
Figure 3 Effect of different concentrations of GQDs on trypsin activity.
Notes: GQDs at different concentrations (150, 125, 100, 75, 50, and 25 µg/mL) were incubated with 1% trypsin in 96-well plates at different time points (2, 5, 10, 15, 30, and 60 min) as shown. (A–D) Comparison of different concentrations of GQDs on trypsin activity over 0–60 min. (E) Influence of the highest concentration of GQDs on trypsin activity compared to the case of GQDs only. Trypsin was highly active at 150 µg/mL concentration of GQDs and slightly active at other concentrations. Fluorescence signals were determined using plate reader at Ex/Em: 355/460 nm. *p<0.05, **p<0.01 and ***p<0.001 GQDs vs GQDs+trypsin. Control wells contained H2O and GQDs, and H2O and trypsin. n.s. denotes not significant.
Abbreviation: GQDs, graphene quantum dots.
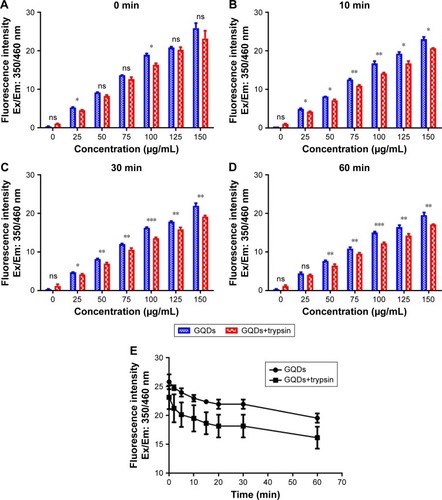
Figure 4 FTIR spectra of trypsin-linked GQDs.
Note: (A) 25, (B) 50, (C) 75, (D) 100, (E) 125, and (F) 150 µg/mL GQDs concentration.
Abbreviations: FTIR, Fourier-transform infrared spectroscopy; GQDs, graphene quantum dots.
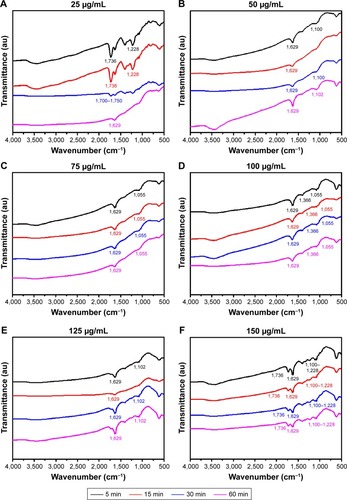
Figure 5 Raman spectra of trypsin-linked GQDs.
Note: (A) 25 and (B) 150 µg/mL.
Abbreviation: GQDs, graphene quantum dots.
Figure 6 Contact angle profiles of trypsin–GQDs interfaces at 25 and 150 µg/mL concentrations of GQDs.
Notes: (A) Water contact angle of interface from 5 to 60 min. (B) DIIO contact angle of interface from 5 to 60 min. DIIO contact was measured to calculate the surface energy of trypsin, GQDs, and their interfaces.
Abbreviations: DIIO, diiodomethane; GQD, grapheme quantum dot.
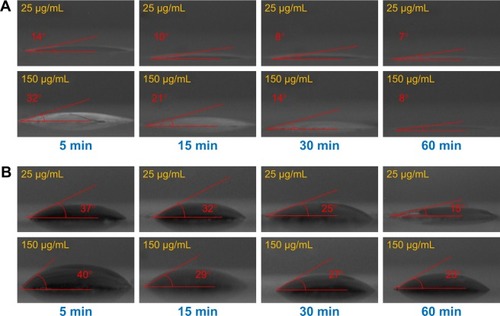
Figure 7 WCA and surface energy profile of GQDs–trypsin interfaces from 0 to 60 min.
Note: (A) WCA, (B) total surface energy, (C) dispersive surface energy, and (D) polar surface energy of 25, 50, 75, 100, 125, and 150 µg/mL concentrations of GQDs treated with trypsin.
Abbreviations: GQDs, graphene quantum dots; WCA, water contact angle.
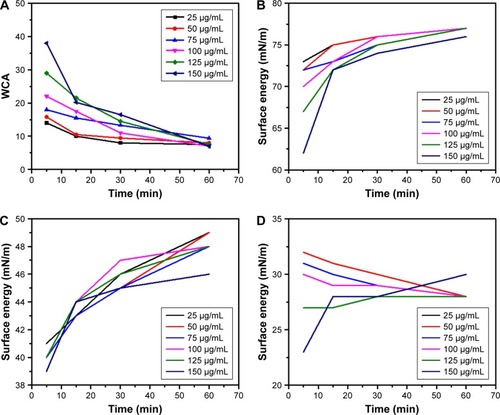
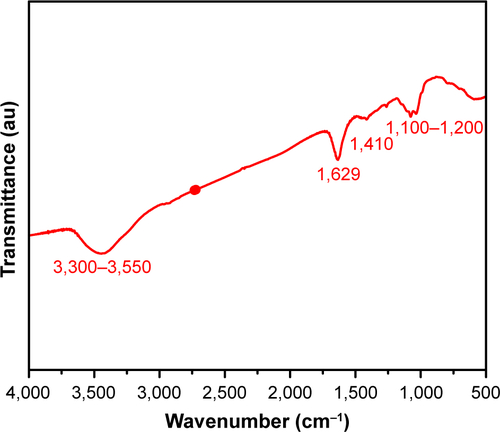
Figure S1 Basic characterization of GQDs.
Notes: (A) Transmission electron microscopy image of GQDs showing their regular diameter, round shape, and spatial distribution. Scale bar: 200 nm. (B) FTIR spectrum of the GQDs showing vibrations of different functional groups. (C) Raman spectrum of the GQDs showing the D (1,355 cm−1) and G peaks (1,580 cm−1). (D) PL spectrum of the GQDs.
Abbreviations: FTIR, Fourier-transform infrared spectroscopy; GQDs, graphene quantum dots; PL, photoluminescence.
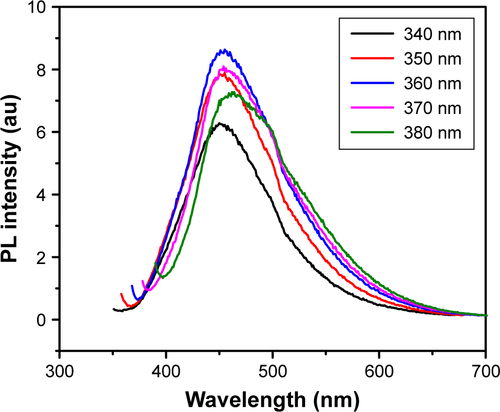
Figure S2 Luminescence property and emission diagram of GQDs.
Notes: PL spectra of GQDs at the excitation wavelength of 340, 350, 360, 370, and 380 nm. The strongest PL emission occurs at 460 nm.
Abbreviations: GQDs, graphene quantum dots; PL, photoluminescence.