Figures & data
Figure 1 The pathway of conversion of CP in the presence of sodium hypochlorite (5% solution).
Abbreviations: CP, cyclophosphamide; CP-TP1-OCl, 4-hydroxy-cyclophosphamide; CP-TP2-OCl, ifosfamide.
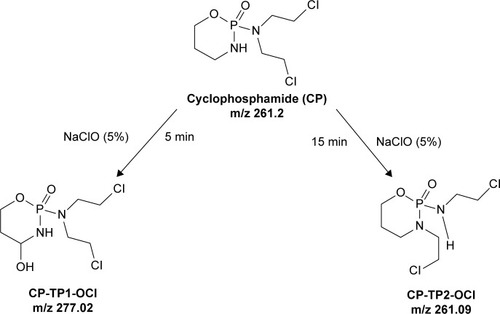
Figure 2 Scheme of degradation CP after reaction with a solution of 0.01 M NaOH.
Abbreviations: CP, cyclophosphamide; CP-TP1-OH, 3-((amino(bis(2-chloroethyl) amino)phosphoryl)oxy) propanoic acid.
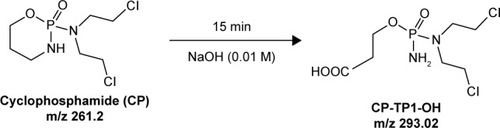
Figure 3 The proposed mechanism of adsorption of CP on a titania surface.
Abbreviation: CP, cyclophosphamide.
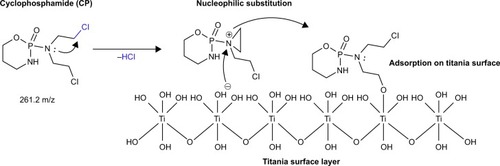
Figure 4 Transformation products formed during CP degradation plotted as a function of the normalized concentration (C/C0) of cyclophosphamide decay.
Note: Transformation products formed during the decontamination time (minutes) in the presence of (A) 5% NaClO agent, (B) 0.01 M NaOH agent, and (C) TiO2.
Abbreviations: CP, cyclophosphamide; CP-TP1-OCl, 4-hydroxy-cyclophosphamide; CP-TP2-OCl, ifosfamide.
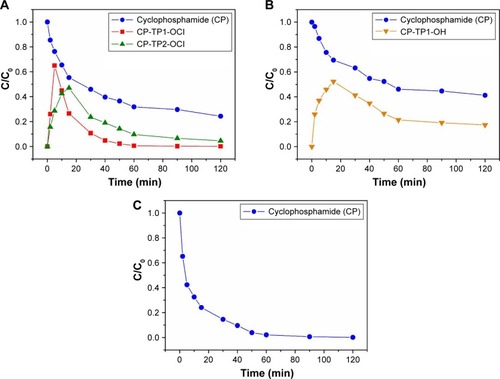
Figure 5 The proposed pathway of conversion of IFOS in the presence of a sodium hydroxide (0.01 M) agent.
Abbreviations: IFOS, ifosfamide; IFOS-TP1-OH, ifosforamide mustard.
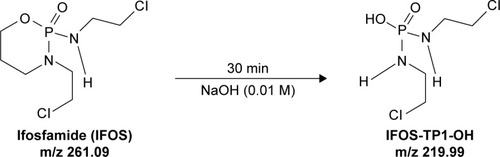
Figure 6 The proposed pathway of adsorption of IFOS on a titania surface.
Abbreviation: IFOS, ifosfamide.
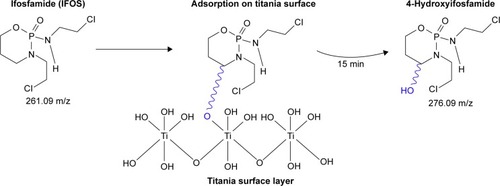
Figure 7 Transformation products formed during IFOS degradation plotted as a function of the normalized concentration (C/C0) of IFOS decay.
Note: Transformation products formed during the decontamination time (minutes) in the presence of (A) 5% NaClO agent, (B) 0.01 M NaOH agent, and (C) TiO2.
Abbreviations: IFOS, ifosfamide; IFOS-TP1-OH, ifosforamide mustard.
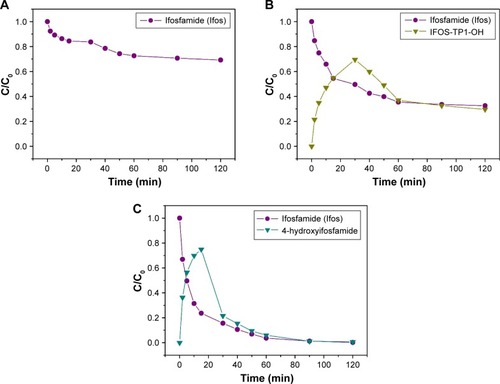
Figure 8 The DRIFTS fingerprint spectra of CP destructive adsorption on the titanium oxide.
Abbreviations: CP, cyclophosphamide; DRIFTS, diffuse reflectance infrared Fourier transform spectroscopy.
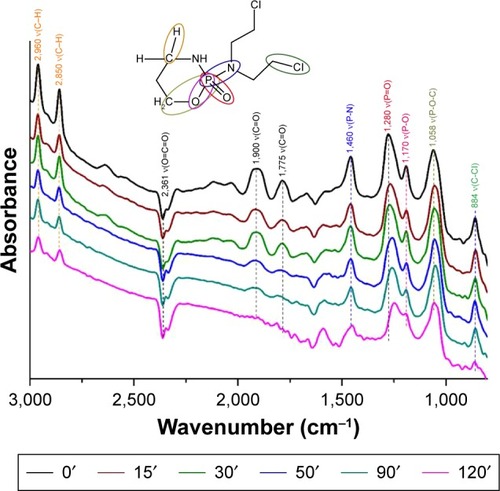
Figure 9 The DRIFTS fingerprint spectra of IFOS destructive adsorption on the titanium oxide.
Abbreviations: IFOS, ifosfamide; DRIFTS, diffuse reflectance infrared Fourier transform spectroscopy.
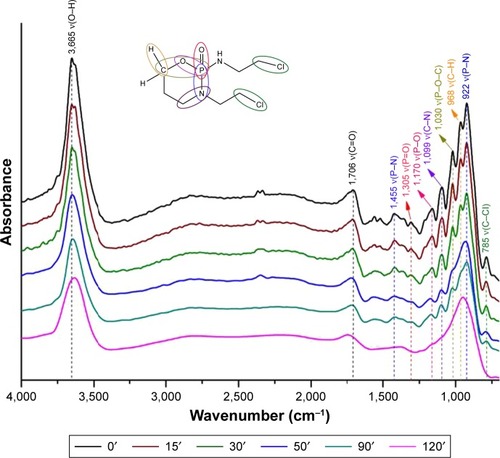
Figure 10 The proposed mechanistic pathway of reactive adsorption of CP on a titania surface with DRIFT characteristics.
Abbreviation: CP, cyclophosphamide.
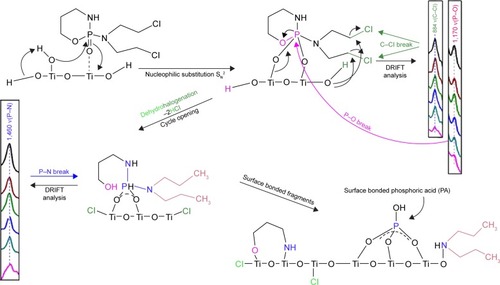
Figure 11 The proposed mechanistic pathway of reactive adsorption of IFOS on a titania surface with DRIFT characteristics.
Abbreviation: IFOS, ifosfamide.
Figure S1 HPLC–MS chromatogram (A) and mass spectra (B) of CP.
Note: Adduction is represented by [M+H]+, formed by the interaction of a molecule with a proton (hydron).
Abbreviations: CP, cyclophosphamide; HPLC–MS, high-performance liquid chromatography–mass spectrometry; NL, intensity of the signal; RT, retention time.
![Figure S1 HPLC–MS chromatogram (A) and mass spectra (B) of CP.Note: Adduction is represented by [M+H]+, formed by the interaction of a molecule with a proton (hydron).Abbreviations: CP, cyclophosphamide; HPLC–MS, high-performance liquid chromatography–mass spectrometry; NL, intensity of the signal; RT, retention time.](/cms/asset/a25029ae-1422-4f83-a77a-6782fe0d9e08/dijn_a_12194008_sf0001_b.jpg)
Figure S2 HPLC–MS chromatogram (A) and mass spectra of the transformation product of CP (B) 4-hydroxycyclophosphamide and (C) 3-(2-chloroethyl)-2-((2-chloroethyl) amino)-1,3,2-oxazaphosphinane 2-oxide (IFOS) from CP in sodium hypochlorite 5% solution (NaClO).
Note: Adduction is represented by [M+H]+, formed by the interaction of a molecule with a proton (hydron).
Abbreviations: CP, cyclophosphamide; HPLC–MS, high-performance liquid chromatography–mass spectrometry; NL, intensity of the signal; RT, retention time.
![Figure S2 HPLC–MS chromatogram (A) and mass spectra of the transformation product of CP (B) 4-hydroxycyclophosphamide and (C) 3-(2-chloroethyl)-2-((2-chloroethyl) amino)-1,3,2-oxazaphosphinane 2-oxide (IFOS) from CP in sodium hypochlorite 5% solution (NaClO).Note: Adduction is represented by [M+H]+, formed by the interaction of a molecule with a proton (hydron).Abbreviations: CP, cyclophosphamide; HPLC–MS, high-performance liquid chromatography–mass spectrometry; NL, intensity of the signal; RT, retention time.](/cms/asset/3235e46c-26ac-4e6f-80be-322ff9afa262/dijn_a_12194008_sf0002_b.jpg)
Figure S3 HPLC–MS chromatogram (A) and mass spectra (B) of transformation product 3-((amino(bis(2-chloroethyl)amino)phosphoryl)oxy) propanoic acid from CP in sodium hydroxide 0.01 M (NaOH) solution.
Note: Adduction is represented by [M+H]+, formed by the interaction of a molecule with a proton (hydron).
Abbreviations: CP, cyclophosphamide; HPLC–MS, high-performance liquid chromatography–mass spectrometry; NL, intensity of the signal; RT, retention time.
![Figure S3 HPLC–MS chromatogram (A) and mass spectra (B) of transformation product 3-((amino(bis(2-chloroethyl)amino)phosphoryl)oxy) propanoic acid from CP in sodium hydroxide 0.01 M (NaOH) solution.Note: Adduction is represented by [M+H]+, formed by the interaction of a molecule with a proton (hydron).Abbreviations: CP, cyclophosphamide; HPLC–MS, high-performance liquid chromatography–mass spectrometry; NL, intensity of the signal; RT, retention time.](/cms/asset/c4497bbe-fc61-4ee3-b5f4-9355f3c2f2b2/dijn_a_12194008_sf0003_b.jpg)
Figure S4 Typical LC–MS chromatogram (A) and mass spectra (B) of IFOS.
Note: Adduction is represented by [M+H]+, formed by the interaction of a molecule with a proton (hydron).
Abbreviations: IFOS, ifosfamide; LC–MS, liquid chromatography–mass spectrometry; NL, intensity of the signal; RT, retention time.
![Figure S4 Typical LC–MS chromatogram (A) and mass spectra (B) of IFOS.Note: Adduction is represented by [M+H]+, formed by the interaction of a molecule with a proton (hydron).Abbreviations: IFOS, ifosfamide; LC–MS, liquid chromatography–mass spectrometry; NL, intensity of the signal; RT, retention time.](/cms/asset/35551bda-f24d-469f-a975-1dc7c678a4f7/dijn_a_12194008_sf0004_b.jpg)
Figure S5 LC–MS chromatogram (A) and mass spectra (B) of the transformation product IFOS-TP1-OH of IFOS in sodium hydroxide 0.01 M (NaOH) solution.
Note: Adduction is represented by [M+H]+, formed by the interaction of a molecule with a proton (hydron).
Abbreviations: IFOS, ifosfamide; IFOS-TPI-OH, 3-((amino(bis(2-chloroethyl)amino)phosphoryl)oxy) propanoic acid; LC–MS, liquid chromatography–mass spectrometry; NL, intensity of the signal; RT, retention time.
![Figure S5 LC–MS chromatogram (A) and mass spectra (B) of the transformation product IFOS-TP1-OH of IFOS in sodium hydroxide 0.01 M (NaOH) solution.Note: Adduction is represented by [M+H]+, formed by the interaction of a molecule with a proton (hydron).Abbreviations: IFOS, ifosfamide; IFOS-TPI-OH, 3-((amino(bis(2-chloroethyl)amino)phosphoryl)oxy) propanoic acid; LC–MS, liquid chromatography–mass spectrometry; NL, intensity of the signal; RT, retention time.](/cms/asset/9e0b2718-34d8-48f2-a11c-f8b05a6b666a/dijn_a_12194008_sf0005_b.jpg)
