Figures & data
Figure 1 Synthesis route of lactoferrin-PEG-DSPE.
Abbreviations: DSPE-PEG2000-MAL, distearoylphosphatidylethanolamine-polyethylene glycol (MW~2 kDa)-maleimide; Lf, lactoferrin; PEG-DSPE, polyethylene glycol-b-distearoylphosphatidylethanolamine.
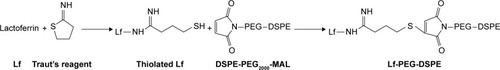
Figure 2 Scheme graph of L/R-T/V-NLCs.
Abbreviations: RGD, arginine–glycine–aspartic acid; L/R-T/V-NLCs, lactoferrin- and arginine–glycine–aspartic acid dual-ligand-comodified, temozolomide and vincristine-coloaded nanostructured lipid carriers.
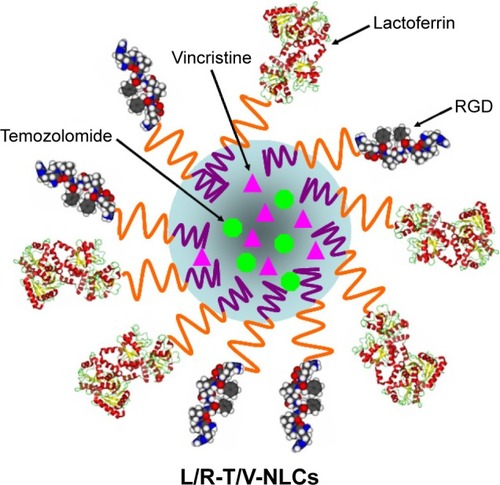
Figure 3 The chemical structure of Lf-PEG-DSPE and 1H-NMR spectroscopy.
Abbreviations: Lf-PEG-DSPE, lactoferrin-PEG-DSPE; L/R-T/V-NLCs, lactoferrin- and arginine–glycine–aspartic acid dual-ligand-comodified, temozolomide and vincristine-coloaded nanostructured lipid carriers.
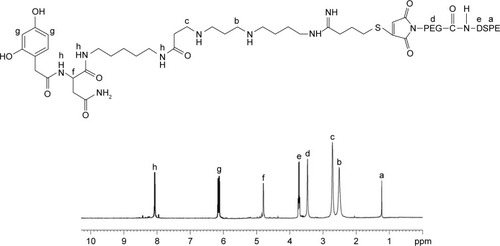
Table 1 Characterization of different vectors
Figure 4 The stability of NLCs evaluated by measuring the NLC sizes (A), PDI (B), TMZ EE (C), and VCR EE (D) for 90 days.
Note: Data represented as mean±SD (n=3).
Abbreviations: EE, encapsulation efficiency; L/R-T/V-NLCs, lactoferrin- and arginine–glycine–aspartic acid dual-ligand-comodified, temozolomide and vincristine-coloaded nanostructured lipid carriers; PDI, polydispersity index; TMZ, temozolomide; VCR, vincristine.
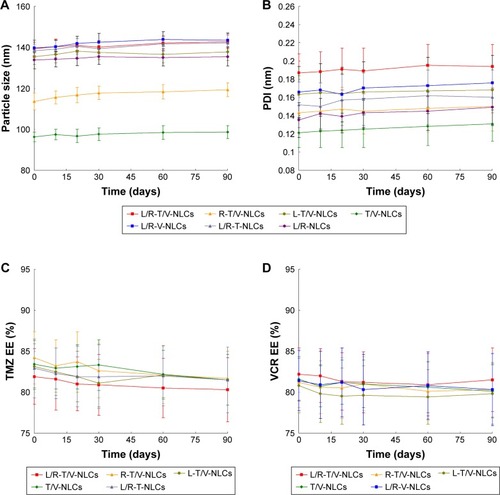
Figure 5 In vitro TMZ (A) and VCR (B) release profiles of all kinds of NLCs.
Note: Data represented as mean±SD (n=3).
Abbreviations: L/R-T/V-NLCs, lactoferrin- and arginine–glycine–aspartic acid dual-ligand-comodified, temozolomide and vincristine-coloaded nanostructured lipid carriers; TMZ, temozolomide; VCR, vincristine.
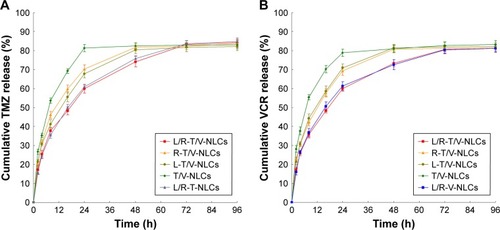
Figure 6 Cellular uptake efficiency of the NLCs on U87 MG cells (A) and A549 MG cells (B).
Note: Data represented as mean±SD (n=3).
Abbreviation: L/R-T/V-NLCs, lactoferrin- and arginine–glycine–aspartic acid dual-ligand-comodified, temozolomide and vincristine-coloaded nanostructured lipid carriers.
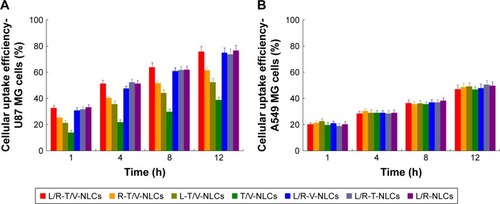
Figure 7 In vitro cytotoxicity of NLCs was evaluated on U87 MG cells (A) and T98G cells (B) by MTT assay and the synergistic effect by measuring CI50 (C).
Note: CI50 indicates the combination index when inhibitory concentration produced 50% cell death.
Abbreviations: L/R-T/V-NLCs, lactoferrin- and arginine–glycine–aspartic acid dual-ligand-comodified, temozolomide and vincristine-coloaded nanostructured lipid carriers; Fa, fraction of affected cells.
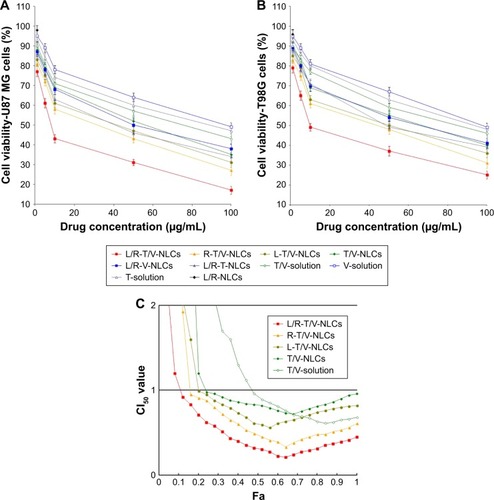
Figure 8 In vivo TMZ tissue distribution of the NLCs and solutions at 10 h (A) and 72 h (B), and VCR tissue distribution of the NLCs and solutions at 10 h (C) and 72 h (D). Data represented as mean±SD (n=8).
Note: There is no data for 0.9% saline group.
Abbreviations: L/R-T/V-NLCs, lactoferrin- and arginine–glycine–aspartic acid dual-ligand-comodified, temozolomide and vincristine-coloaded nanostructured lipid carriers; TMZ, temozolomide; VCR, vincristine.
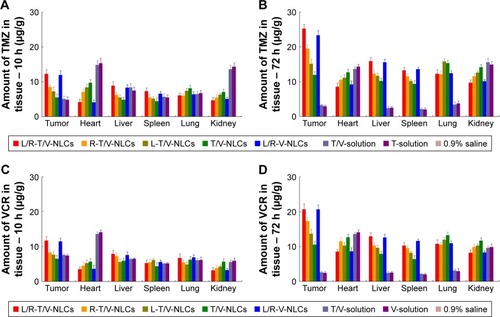
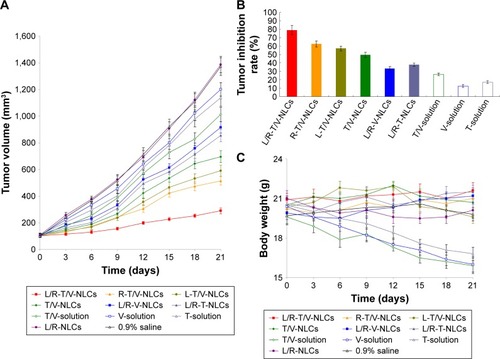
Figure 9 In vivo antitumor efficiency of NLCs evaluated using a xenograft nude mouse model.
Notes: Tumor volume (A); tumor inhibition rate (B); body weight (C). Data represented as mean±SD (n=8).
Abbreviation: L/R-T/V-NLCs, lactoferrin- and arginine–glycine–aspartic acid dual-ligand-comodified, temozolomide and vincristine-coloaded nanostructured lipid carriers.