Figures & data
Figure 1 Schematic illustration of RIPL-NLC preparation.
Notes: (A) Representative structure of DTX-RIPL-NLC. (B) Schematic procedure for preparing RIPL-NLC.
Abbreviations: DP2M, distearoyl phosphatidylethanolamine-polyethylene glycol2000-maleimide; DTX, docetaxel; DTX-RIPL-NLC, docetaxel-loaded RIPL peptide-conjugated nanostructured lipid carrier; DW, distilled water; NLC, nanostructured lipid carrier; PVA, polyvinylalcohol; RIPL-NLC, RIPL peptide-conjugated nanostructured lipid carrier.
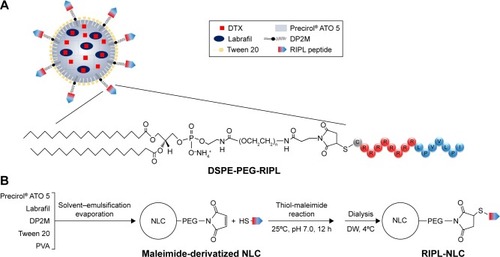
Table 1 Physicochemical characteristics of NLCs
Figure 2 Characterization of RIPL-NLC.
Notes: (A) Time-dependency of the thiol-maleimide reaction. (B) Comparison of the calculated FALT of NLC and RIPL-NLC. (C) TEM images of DTX-NLC and DTX-RIPL-NLC. (D) DSC thermograms of DTX, Precirol® ATO 5, DTX-NLC, and DTX-RIPL-NLC. Data are mean ± SD (n=3).
Abbreviations: DSC, differential scanning calorimetry; DTX-NLC, DTX-loaded nanostructured lipid carrier; DTX-RIPL-NLC, docetaxel-loaded RIPL peptide-conjugated nanostructured lipid carrier; FALT, fixed aqueous layer thickness; NLC, nanostructured lipid carrier; RIPL-NLC, RIPL peptide-conjugated nanostructured lipid carrier; TEM, transmission electron microscopy.
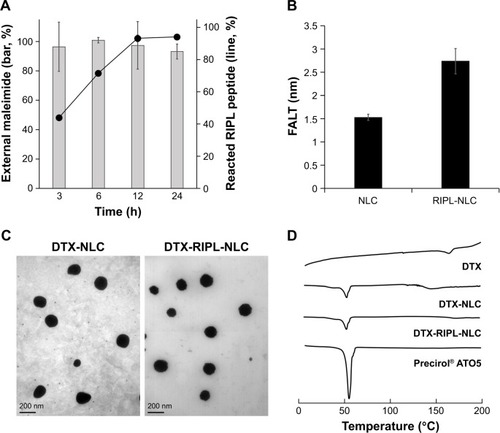
Figure 3 Colloidal stability of DTX-loaded NLCs in different media.
Notes: Samples were incubated at 37°C. Particle size was determined by dynamic light scattering. Data are mean ± SD (n=3).
Abbreviations: DTX, docetaxel; DW, distilled water; PBS, phosphate-buffered saline; RPMI, Roswell Park Memorial Institute 1640 medium; DTX-NLC, docetaxel-loaded nanostructured lipid carrier; DTX-RIPL-NLC, docetaxel-loaded RIPL peptide-conjugated nanostructured lipid carrier.
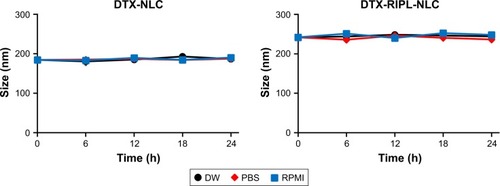
Figure 4 In vitro drug release profile of various DTX formulations.
Note: Data are mean ± SD (n=3).
Abbreviations: DTX, docetaxel; DTX-Sol, docetaxel solution; DTX-NLC, docetaxel-loaded nanostructured lipid carrier; DTX-RIPL-NLC, docetaxel-loaded RIPL peptide-conjugated nanostructured lipid carrier.
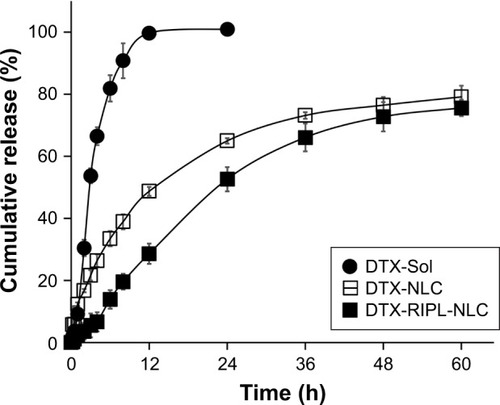
Table 2 In vitro release kinetic parameters of various DTX formulations
Figure 5 The qualitative and quantitative analysis of the cellular uptake of DiI-loaded formulations.
Notes: (A) Fluorescence microscopy images of various cell lines incubated with DiI-Sol, DiI-NLC, and DiI-RIPL-NLC at 37°C for 2 h. Concentration of DiI was 200 ng/mL. White scale bar represents 100 µm. (B) Flow cytometry histogram shows the treatment effect: untreated cells (purple); cells treated with DiI-Sol (red), DiI-NLC (green), and DiI-RIPL-NLC (black). (C) The relative ratio of MFI values in different treatments: DiI-NLC versus DiI-Sol; DiI-RIPL-NLC versus DiI-NLC. Data are mean ± SD (n=3).
Abbreviations: DiI, 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate; DiI-Sol, DiI solution; DiI-NLC, DiI-loaded nanostructured lipid carrier; DiI-RIPL-NLC, DiI-loaded RIPL peptide-conjugated nanostructured lipid carrier; Hpn, Hepsin; MFI, mean fluorescence intensity.
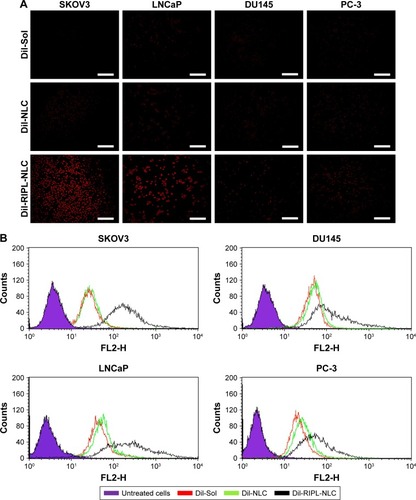
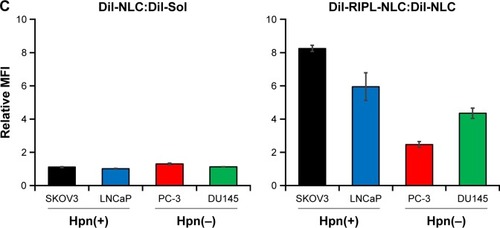
Figure 6 Confocal microscopic images with orthogonal views of SKOV3 and PC-3 cells.
Notes: Cells incubated with DiI-RIPL-NLC for 0 (immediately after the treatment), 1, 2, and 4 h. The nucleus was stained with DAPI for blue fluorescence and merged with red fluorescence of DiI distributed in the cytoplasm. The positions of the section plane are indicated by colored lines; XY plane (blue), XZ plane (green), YZ plane (red). The white scale bar represents 20 µm.
Abbreviations: DAPI, 4′,6-diamino-2-phenylindole; DiI, 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate; DiI-Sol, DiI solution; DiI-NLC, DiI-loaded nanostructured lipid carrier; DiI-RIPL-NLC, DiI-loaded RIPL peptide-conjugated nanostructured lipid carrier; Hpn, hepsin.
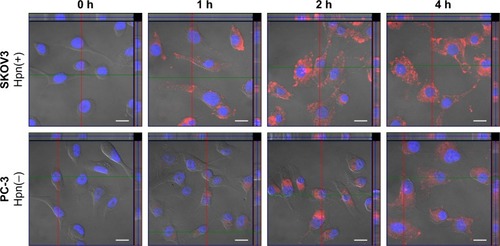
Figure 7 The cytotoxicity of DTX-free formulations (upper panels) and DTX-loaded formulations (lower panels) on SKOV3, LNCaP, DU145, and PC-3 cells by WST-1 assay.
Notes: Data are mean ± SD (n=3). Statistical analysis was performed by using Student’s t-test (*P<0.05 versus DTX-Sol; #P<0.05 versus DTX-NLC).
Abbreviations: DTX, docetaxel; DTX-Sol, docetaxel solution; DTX-NLC, docetaxel-loaded nanostructured lipid carrier; DTX-RIPL-NLC, docetaxel-loaded RIPL peptide-conjugated nanostructured lipid carrier; Conc, concentation.
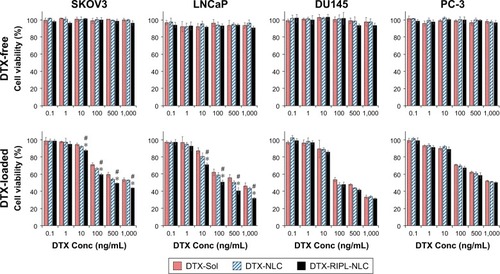
Figure 8 In vivo antitumor efficacies in SKOV3-bearing BALB/c nude mice treated with different formulations.
Notes: Animals (n=5) received 10 mg DTX/kg intratumorally on day 0, 7, and 14, a total of three injections per mouse as indicated by arrows. (A) Changes in tumor volume for 6 weeks post-administration. (B) Body weight changes for 6 weeks post-administration. (C) Morphology of excised xenograft tumors at the end of the study (day 42). Black bar indicates 30 mm. Statistical analysis was performed using Student’s t-test (*P<0.05 versus saline; #P<0.05 versus DTX-Sol).
Abbreviations: DTX, docetaxel; DTX-Sol, docetaxel solution; DTX-NLC, docetaxel-loaded nanostructured lipid carrier; DTX-RIPL-NLC, docetaxel-loaded RIPL peptide-conjugated nanostructured lipid carrier.
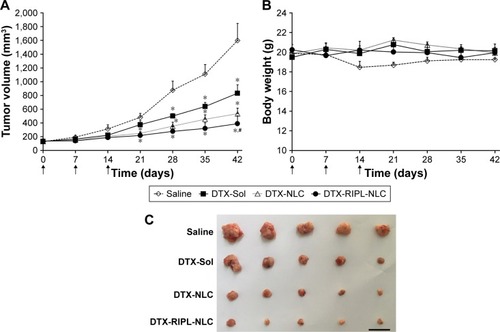
Table 3 Antitumor efficacy of treatment groups in a SKOV3 cell-bearing xenograft mouse model
