Figures & data
Table 1 Compositions of raloxifene transdermal formulations
Figure 1 Particle size frequencies and images of raloxifene transdermal formulations with or without menthol.
Notes: Particle size frequencies of Ral-MPs (A), mRal-MPs (B), Ral-NPs (C), and mRal-NPs (D) by SALD-71000. Means ± SD and particle size frequencies of Ral-NPs (E) and mRal-NPs (F) by NANOSIGHT LM10. Means ± SE and SPM images of Ral-NPs (G) and mRal-NPs (H). The raloxifene particles remained in the nano size range following bead mill treatment. The particle size frequencies showed no difference between Ral-NPs and mRal-NPs.
Abbreviations: mRal-MPs, transdermal formulation containing raloxifene microparticles and menthol; mRal-NPs, transdermal formulation containing raloxifene nanoparticles and menthol; Ral-MPs, transdermal formulation containing raloxifene microparticles; Ral-NPs, transdermal formulation containing raloxifene nanoparticles; SE, standard error of the mean.
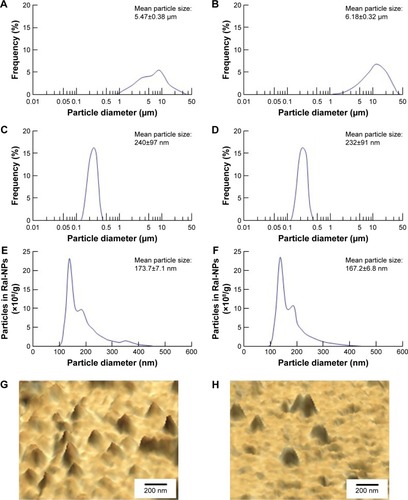
Figure 2 Stability of raloxifene transdermal formulations 15 and 30 days after bead mill treatment.
Notes: Particle size frequencies of Ral-NPs 15 days (A) and 30 days (B) after bead mill treatment. Particle size frequencies of mRal-NPs 15 days (C) and 30 days (D) after bead mill treatment. The particle size was measured by NANOSIGHT LM10. SPM images of Ral-NPs (E) and mRal-NPs (F) 30 days after bead mill treatment. (G) Changes in the raloxifene contents of transdermal formulations after bead mill treatment. The data represent the means ± SE, n=10. The transdermal formulations containing raloxifene nanoparticles were stable because no drug precipitation or degradation was observed during 30 days after preparation.
Abbreviations: mRal-MPs, transdermal formulation containing raloxifene microparticles and menthol; mRal-NPs, transdermal formulation containing raloxifene nanoparticles and menthol; Ral-MPs, transdermal formulation containing raloxifene microparticles; Ral-NPs, transdermal formulation containing raloxifene nanoparticles; SE, standard error of the mean.
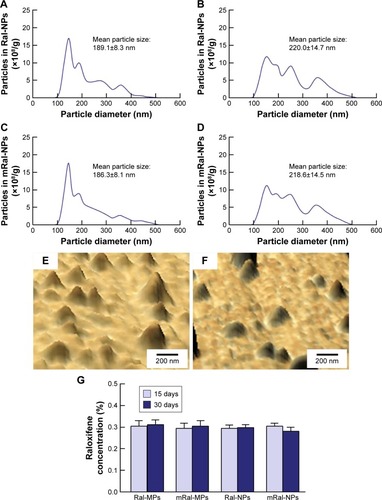
Figure 3 Drug release from 0.3% raloxifene transdermal formulations through a 450 nm pore membrane.
Notes: (A) Release of raloxifene from Ral-MPs and mRal-MPs. (B) Release of raloxifene from Ral-NPs and mRal-NPs. Changes in AUCRelease (C) and nanoparticle number (D) by the application of raloxifene transdermal formulation. Particle size frequency in the Franz diffusion cell (reservoir chamber) after the application of Ral-NPs (E) and mRal-NPs (F). The data represent the means ± SE, n=6–12. *P<0.05 vs Ral-MPs. **P<0.05 vs mRal-MPs. Raloxifene release from Ral-NPs was significantly higher than that from Ral-MPs, and the raloxifene released from Ral-NPs remained in the nanoparticle state. Moreover, the addition of menthol did not affect raloxifene release from the transdermal formulation.
Abbreviations: mRal-MPs, transdermal formulation containing raloxifene microparticles and menthol; mRal-NPs, transdermal formulation containing raloxifene nanoparticles and menthol; Ral-MPs, transdermal formulation containing raloxifene microparticles; Ral-NPs, transdermal formulation containing raloxifene nanoparticles; SE, standard error of the mean.
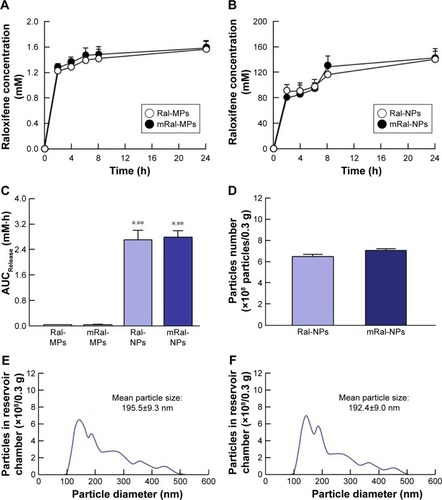
Figure 4 Skin penetration of raloxifene released from 0.3% raloxifene transdermal formulations.
Notes: (A) Skin penetration of Ral-MPs and mRal-MPs. (B) Skin penetration of Ral-NPs and mRal-NPs. (C) Penetration of Ral-MPs and Ral-NPs through skin pretreated with menthol. (D) Penetration of Ral-NPs and mRal-NPs through skin from which the stratum corneum was removed. The data represent the means ± SE, n=5–8. *P<0.05 vs Ral-MPs for each category. Menthol attenuated the barrier function of the stratum corneum and permitted the penetration of raloxifene nanoparticles through stratum corneum.
Abbreviations: mRal-MPs, transdermal formulation containing raloxifene microparticles and menthol; mRal-NPs, transdermal formulation containing raloxifene nanoparticles and menthol; Ral-MPs, transdermal formulation containing raloxifene microparticles; Ral-NPs, transdermal formulation containing raloxifene nanoparticles; SE, standard error of the mean.
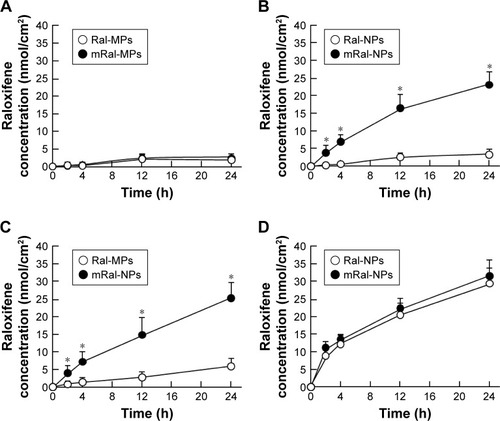
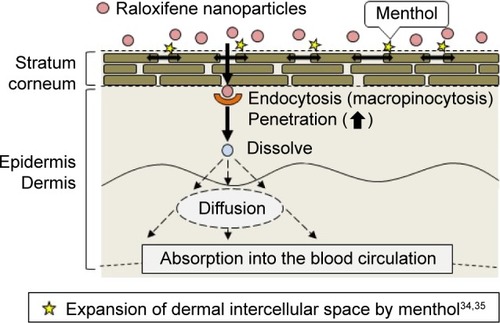
Figure 5 Involvement of endocytosis in the skin penetration of raloxifene released from 0.3% mRal-NPs.
Notes: (A) Effect of endocytosis inhibitors on the penetration in mRal-NPs through the skin. (B) Changes in drug penetration levels (AUCPenetration) of mRal-NPs through endocytosis inhibitor-treated skin. The skin samples were pretreated for 1 h with 0.5% DMSO (vehicle), 54 µM nystatin, 40 µM dynasore, 2 µM rottlerin, or 10 µM cytochalasin D. The data represent the means ± SE, n=5–8. *P<0.05 vs vehicle for each category. Macropinocytosis is related to the transdermal penetration of raloxifene nanoparticles.
Abbreviations: mRal-MPs, transdermal formulation containing raloxifene microparticles and menthol; mRal-NPs, transdermal formulation containing raloxifene nanoparticles and menthol; Ral-MPs, transdermal formulation containing raloxifene microparticles; Ral-NPs, transdermal formulation containing raloxifene nanoparticles; SE, standard error of the mean; DMSO, dimethyl sulfoxide.
Table 2 Pharmacokinetic parameters for the percutaneous absorption of mRal-NPs
Figure 6 Changes in plasma raloxifene concentrations after the application of 0.3% raloxifene transdermal formulations.
Notes: (A) Plasma raloxifene concentrations after the application of Ral-MPs and mRal-MPs. (B) Plasma raloxifene concentrations after a single application of Ral-NPs or mRal-NPs. (C) AUCPlasma in rats treated with raloxifene transdermal formulations. (D) Plasma raloxifene concentrations after the repetitive application of mRal-NPs. Solid lines represent the fitted curves for multiple applications of mRal-NPs (0.3 g/day, interval 24 h). The data were estimated according to EquationEquations 1(1) –Equation3
(3) and they represent the means ± SE, n=5–6. *P<0.05 vs Ral-MPs for each category. **P<0.05 vs mRal-MPs for each category. ***P<0.05 vs mRal-NPs for each category. The plasma raloxifene concentration in rats treated with transdermal formulations containing raloxifene nanoparticles was enhanced by the presence of menthol in the formulation.
Abbreviations: mRal-MPs, transdermal formulation containing raloxifene microparticles and menthol; mRal-NPs, transdermal formulation containing raloxifene nanoparticles and menthol; Ral-MPs, transdermal formulation containing raloxifene microparticles; Ral-NPs, transdermal formulation containing raloxifene nanoparticles; SE, standard error of the mean.
 and they represent the means ± SE, n=5–6. *P<0.05 vs Ral-MPs for each category. **P<0.05 vs mRal-MPs for each category. ***P<0.05 vs mRal-NPs for each category. The plasma raloxifene concentration in rats treated with transdermal formulations containing raloxifene nanoparticles was enhanced by the presence of menthol in the formulation.Abbreviations: mRal-MPs, transdermal formulation containing raloxifene microparticles and menthol; mRal-NPs, transdermal formulation containing raloxifene nanoparticles and menthol; Ral-MPs, transdermal formulation containing raloxifene microparticles; Ral-NPs, transdermal formulation containing raloxifene nanoparticles; SE, standard error of the mean.](/cms/asset/3f2cb56a-dc76-480d-9885-4b3cb26fd2ff/dijn_a_12194201_f0006_c.jpg)
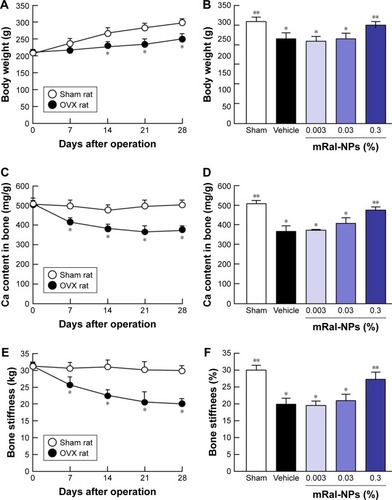
Figure 7 Changes in body weight, Ca content of the bone, and bone stiffness in OVX rats treated with 0.003%, 0.03%, or 0.3% mRal-NPs, respectively.
Notes: Changes in body weight (A), Ca content of the bone (C), and bone stiffness (E) 0–28 days after ovariectomy. Effects of the application of raloxifene transdermal formulations on body weight (B), Ca content of the bone (D), and bone stiffness (F) in OVX rats 28 days after ovariectomy. The raloxifene transdermal formulations were applied for 28 days (0.3 g, once a day) after ovariectomy. The data represent the means ± SE, n=5–8. *P<0.05 vs sham for each category. **P<0.05 vs vehicle for each category. mRal-NPs attenuated the decrease in body weight, Ca content of the bone, and stiffness of the bone of OVX rats.
Abbreviations: mRal-MPs, transdermal formulation containing raloxifene microparticles and menthol; mRal-NPs, transdermal formulation containing raloxifene nanoparticles and menthol; OVX rat, ovariectomized rat; Ral-MPs, transdermal formulation containing raloxifene microparticles; Ral-NPs, transdermal formulation containing raloxifene nanoparticles; SE, standard error of the mean.