Figures & data
Figure 1 Schematic presentation of ApoA-I peptide interaction with lipids. (A) Synthetic HDL (sHDL) particle assembly process: 1) ApoA-I peptides adsorb to the surface of liposomes and partition into the phospholipid bilayer; 2) Bilayer solubilization and assembly of sHDL occur after a critical peptide to lipid ratio is reached. (B) Lipoprotein remodeling in vivo. Upon administration, sHDL interacts with endogenous HDL in bloodstream by exchanging protein and phospholipid components. This interaction results in the formation of lipid-poor endogenous ApoA-I, lipid poor ApoA-I synthetic peptide, and HDL particles containing protein and peptide components.
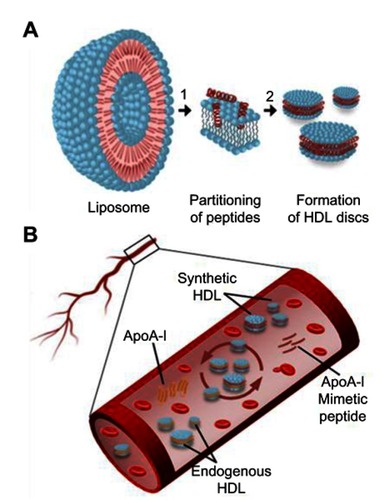
Figure 2 Kinetics of synthetic HDL (sHDL) assembly examined by the decrease in UV600nm turbidity of large unilamellar vesicle (LUV) solutions during incubation with 22A peptide. Liposomes at 5 mg/mL concentration of egg sphingomyelin (eSM) (A/C) and 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC) (B/D) were incubated with 5 mg/mL 22A peptide for 24 hrs at three different temperatures. sHDL assembly during the first hour of incubation is shown in the figure inserts (C and D).
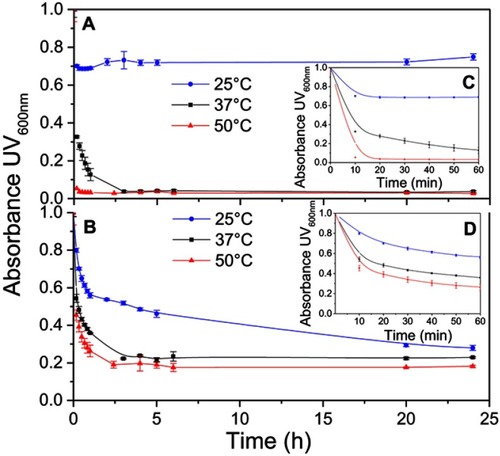
Figure S1 Lipid solubilization assay for egg sphingomyelin (eSM, red) and synthetic sphingomyelin (sSM, blue). Kinetics of sHDL assembly examined by the decrease in UV600 nm turbidity of LUV solutions during incubation with 22A peptide. Liposomes at a concentration of 5 mg/mL were incubated with 5 mg/mL 22A peptide for 1 hr at 25°C (A), 37°C (B), and 50°C (C).
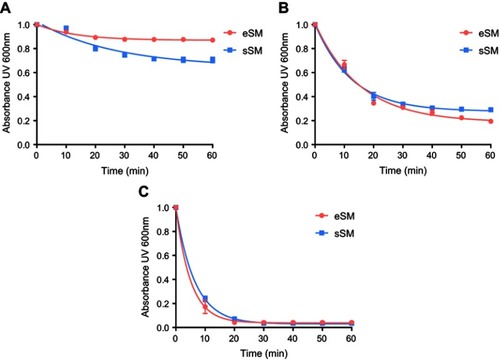
Figure S2 Lipid solubilization assay at different concentrations. Kinetics of sHDL assembly examined by the decrease in UV600 nm turbidity of egg sphingomyelin (eSM) large unilamellar vesicles (LUVs) incubated with 22A peptide at 1:1 weight ratio and 1, 5, and 10 mg/mL peptide concentrations. Liposomes were incubated with 22A peptide for 1 hr at 37°C (A) and 50°C (B), respectively. Data are represented as mean±SD, and were analyzed via nonlinear fit-one phase decay by Graphpad Prism.
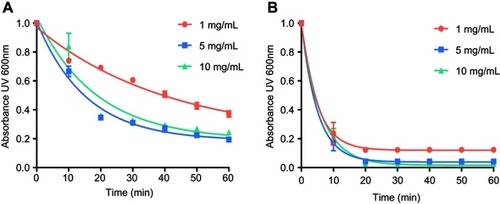
Figure 3 Dynamic light scattering (DLS) volume distributions of particle sizes for egg sphingomyelin (eSM)-based (A) and 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC)-based (C) sHDL formed after 24 hr incubation of lipid large unilamellar vesicles (LUVs) and 22A peptide at different temperatures. The homogeneity of particle size assessed by gel permeation chromotography (GPC) following 24-hr incubations of 22A peptide with eSM (B) and POPC (D) LUVs at different temperatures.
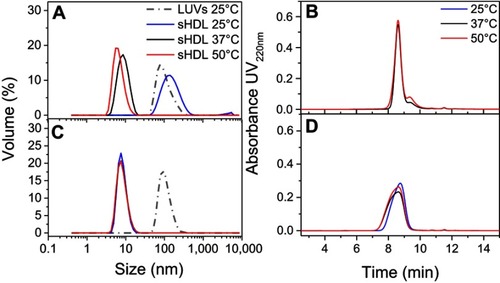
Figure S3 Dynamic light scattering (DLS) intensity distributions of particle sizes for egg sphingomyelin (eSM)- (A) and 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC)-based (B) sHDL following 24-h incubation of lipid large unilamellar vesicles (LUVs) and 22A peptide at different temperatures.
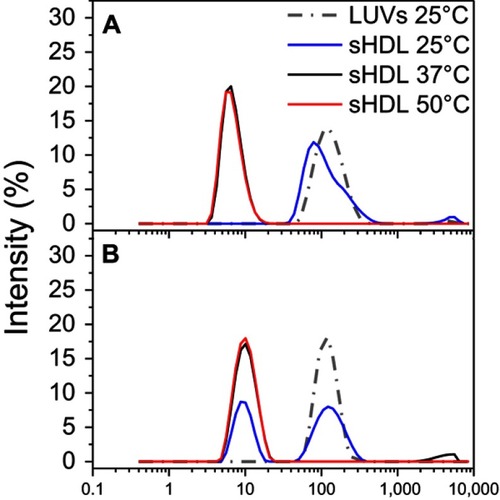
Figure S4 Gel permeation chromotography (GPC) profile of 1 mg/mL 22A peptide solutions immediately after preparation and following 1-hr incubation at 25°C, 37°C, and 50°C.
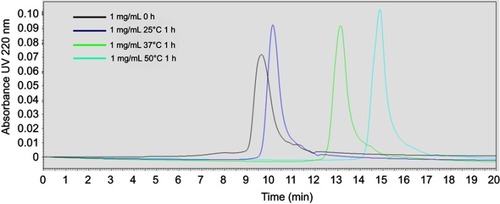
Figure S5 Dynamic light scattering (DLS) profiles of 1, 5, and 10 mg/mL solutions of 22A peptide before and after 1-hr incubation at 25°C, 37°C, and 50°C.
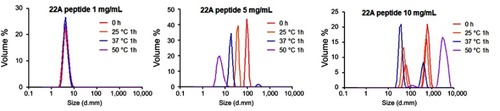
Figure 4 Transmission electron microscopy (TEM) images of large unilamellar vesicles (LUVs) of egg sphingomyelin (eSM) (A) and 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC) (D); sHDL nanoparticles formed following 1-hr incubations of 22A peptide with eSM (B) and POPC (E) LUVs at 37°C and with eSM (C) and POPC (F) LUVs at 50°C.
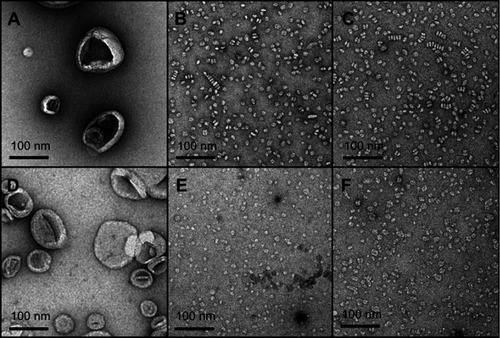
Figure 5 Schematic diagram (A) of the experimental setup for sum frequency generation (SFG) spectroscopy analysis of lipid bilayer-peptide interactions; SFG O-H/N-H stretching signals collected from 22A peptide associated with egg sphingomyelin (eSM) at 20°C (B), 37°C (C), and 50°C (D); SFG amide range stretching signals collected from 22A peptide associated with eSM at 37°C (E) and 50°C (F); ppp refers to polarization combination of p-polarized SFG, p-polarized visible, p-polarized IR and ssp refers to s-polarized SFG, s-polarized visible, p-polarized IR.
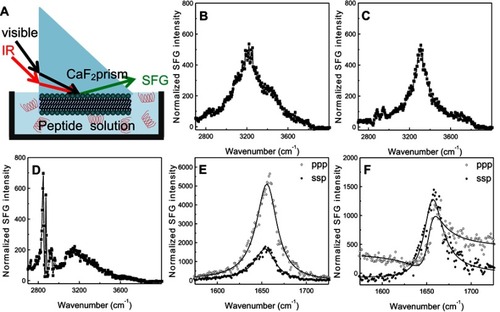
Figure S6 Sum frequency generation (SFG) O-H/N-H stretching signals collected from 22A peptide associated with model lipid membranes of egg sphingomyelin (eSM) at 25°C (A), 37°C (B), and 50°C (C) and 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC) at 20°C (D), 37°C (E), and 50°C (F).
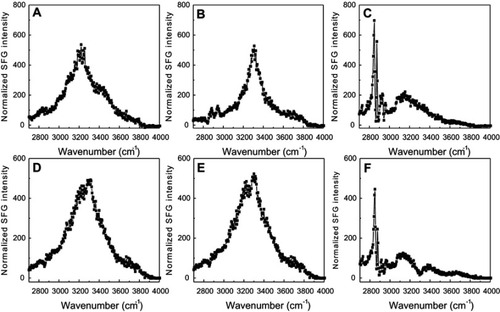
Figure S7 The ssp and ppp sum frequency generation (SFG) amide I spectra collected from the 22A peptide associated with model lipid membranes of egg sphingomyelin (eSM) at 25°C (A), 37°C (B), and 50°C (C) and 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC) at 25°C (D), 37°C (E), and 50°C (F). ppp refers to polarization combination of p-polarized SFG, p-polarized visible, p-polarized IR and ssp refers to s-polarized SFG, s-polarized visible, p-polarized IR.
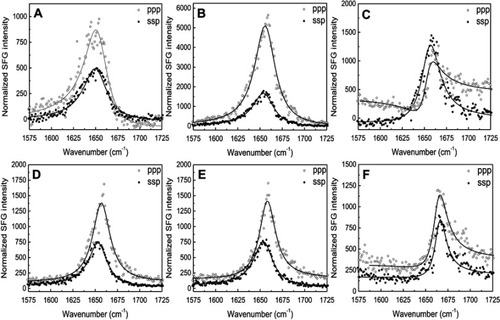
Figure S8 Attenuated total reflection-Fourier transform infrared (ATR-FTIR) spectra of egg sphingomyelin (eSM) bilayer only and 22A peptide with eSM difference spectra at three different temperatures. (A) ATR-FTIR spectra of 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC) bilayer only and 22A peptide with POPC difference spectra at three different temperatures (B).
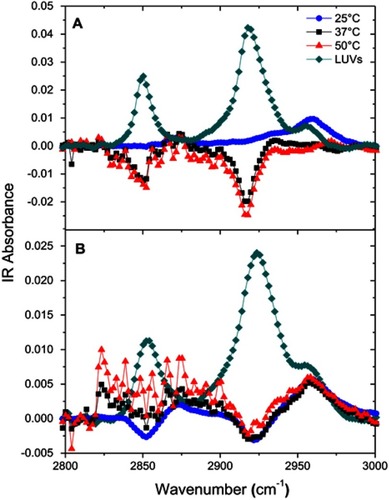
Figure 6 Pharmacokinetics of 22A peptide following administration of assembled egg sphingomyelin (eSM) and 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC)-based sHDL to Sprague-Dawley rats at 100 mg/kg by intravenous injection. Peptide serum levels were determined by liquid chromotography-mass spectroscopy (LC-MS) (N=3 animals/group, mean±SEM).
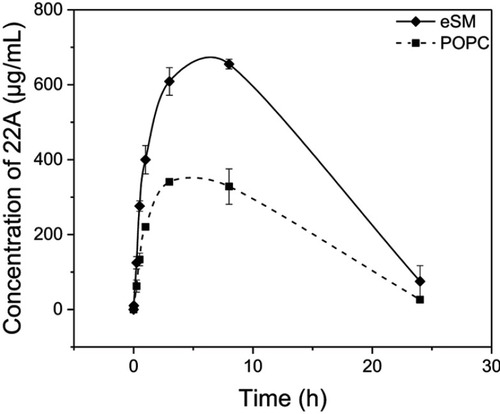
Table S1 Size and purity characterization of POPC- and eSM-based sHDL assembled by 22A peptide and LUV incubations
