Figures & data
Figure 1 The NOA86 (A–C) and PUA (D–F) substrates were coated with silicone and carved to create 800 nm-wide nanogrooves for nanotopographic cues.
Note: SEM images show 800 nm grooves on the plates (B and E are 10,000× magnification images and C and F are 50,000× magnification images).
Abbreviations: NOA86, Norland Optical Adhesive 86; PUA, polyurethane; SEM, scanning electron microscopy.
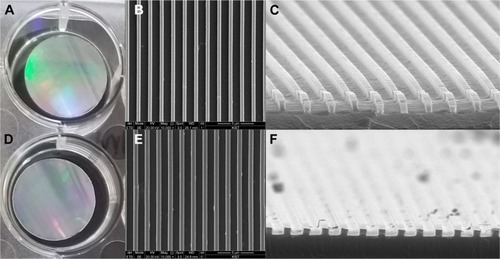
Figure 2 TDSCs were seeded and cultured on the PUA substrate with 19.8 MPa stiffness and 800 nm-wide nanogrooves, NOA86 substrate with 2.4 GPa stiffness and 800 nm-wide nanogrooves, and NOA86 substrate with 2.4 GPa stiffness and flat surface.
Note: Irrespective of age and pathological status, TDSCs cultured on the 800 nm NOA86 and 800 nm PUA substrates were well aligned along the grooves while cells cultured on the flat NOA86 were not.
Abbreviations: NOA86, Norland Optical Adhesive 86; PUA, polyurethane; TDSCs, tendon-derived stem cells.
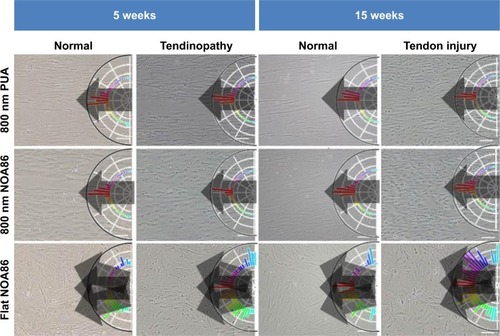
Figure 3 Expression of Col I and Col III collagen was observed in the TDSCs extracted from 5-week normal and 5-week tendinopathy models cultured on the 800 nm NOA86 (2.4 GPa), flat NOA86, and 800 nm PUA (19.8 MPa) substrates.
Notes: In the 5-week normal and 5-week tendinopathic conditions, there was no difference in expression of type I collagens according to nanotopographic cues and substrate stiffness. Expression of Col III collagen in 5-week normal condition was slightly higher in flat NOA86 than in 800 nm PUA or in 800 nm NOA86, while this expression in 5-week tendinopathic conditions was slightly higher in 800 nm NOA86 than in 800 nm PUA or in flat NOA86.
Abbreviations: Col I, type I collagen; Col III, type III collagen; NOA86, Norland Optical Adhesive 86; PUA, polyurethane; TDSCs, tendon-derived stem cells.
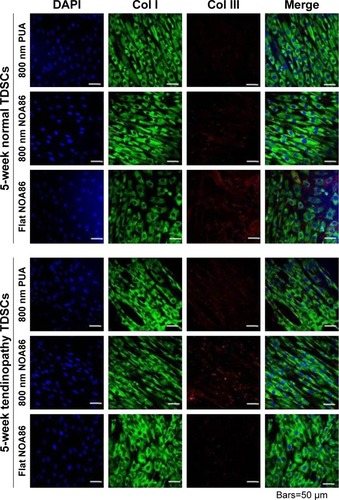
Figure 4 Expression of type I and type III collagen was observed in the TDSCs extracted from 15-week normal and 15-week tendon injury models cultured on the 800 nm NOA86 (2.4 GPa), flat NOA86, and 800 nm PUA (19.8 MPa) substrates.
Notes: In the 15-week normal tendon model, expression of type III collagen was high in TDSCs cultured on the 800 nm NOA86 substrates. In the 15-week tendon injury model, expression of type III collagen was similar irrespective of nanotopographic cues and substrate stiffness. The expression of type I collagen was not different between nanotopographic cues and substrate stiffness in the 15-week normal and tendon injury models.
Abbreviations: Col I, type I collagen; Col III, type III collagen; NOA86, Norland Optical Adhesive 86; PUA, polyurethane; TDSCs, tendon-derived stem cells.
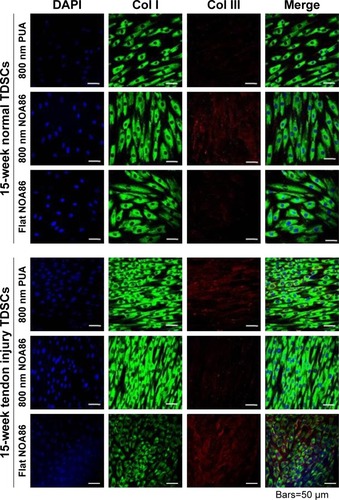
Figure 5 Gene expression of scleraxis increased in TDSCs cultured on the flat NOA86 substrate in the 5-week normal tendon model (*P<0.01).
Notes: In the 15-week normal tendon model, scleraxis was highly expressed in TDSCs cultured on the 800 nm and flat NOA86 substrate (*P<0.01). However, the gene expression was not significantly different between the substrates in the 5-week tendinopathy and 15-week tendon injury models.
Abbreviations: NOA86, Norland Optical Adhesive 86; PUA, polyurethane; TDSCs, tendon-derived stem cells.
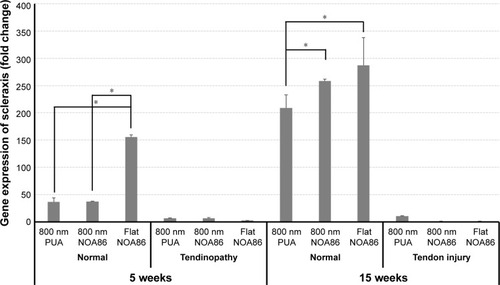
Figure S1 H&E staining of the left Achilles tendon confirmed the pathological findings of tendinopathy and tendon injury models.
Note: Five-week-old tendinopathy model showed an irregular pattern of collagen fibers with multiple lipid vacuoles (A), and the 15-week-old tendon injury model showed a thickened irregular pattern of collagen fibers with abundant polymorphic nuclear cells (B).
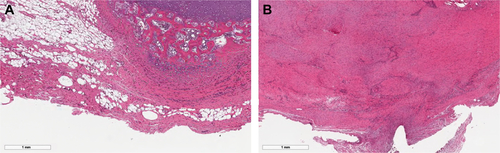
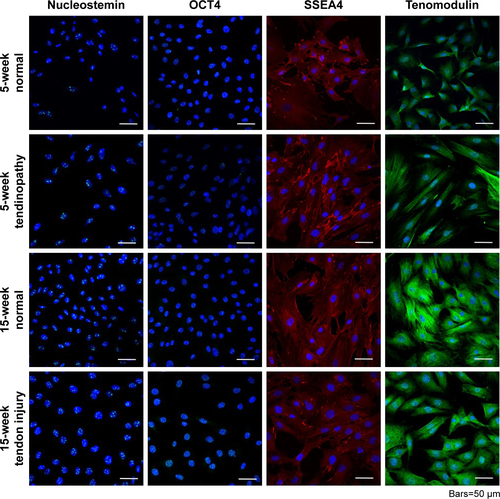
Figure S2 Isolation of TDSCs was validated by identifying cells that positively stained for nucleostemin, OCT4, SSEA4, and tenomodulin. Each TDSC cell line was successfully differentiated into osteogenic, adipose, and chondrogenic cell lines, demonstrating their multipotent capacity.
Abbreviation: TDSCs, tendon-derived stem cells.