Figures & data
Figure 1 1H NMR spectra of DOX (in DMSO-d6), PEG-b-PLL (in D2O), PEG-b-P(LL-g-ss-DOX) (in DMSO-d6), and PEG-b-P(LL-g-ss-DOX)-(LL-g-DMA) (in DMSO-d6).
Abbreviations: DMSO-d6, deuterated dimethyl sulfoxide; DOX, doxorubicin; NMR, nuclear magnetic resonance; PEG-b-PLL, poly(ethylene glycol)-b-poly(L-lysine).
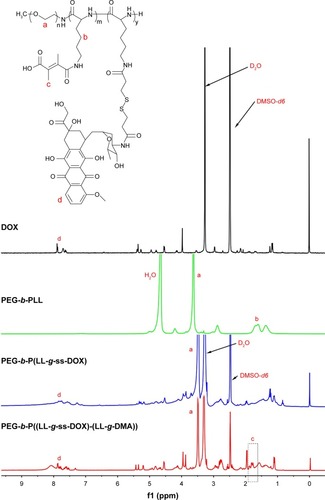
Table 1 Characterization of DOX- and TRI-coloaded nanoparticles
Figure 2 TEM image and size of DA-ss-DT (A), DA-cc-DT (B), SA-ss-DT (C), and P-ss-DT (D). Scale bar=100 nm.
Abbreviation: TEM, transmission electron microscopy.
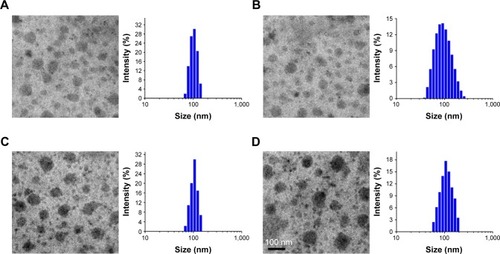
Figure 3 Zeta potential of DA-ss-DT, DA-cc-DT, SA-ss-DT, and P-ss-DT at pH 6.5 (A) and 7.4 (B) at different incubation times (mean±SD, n=3).
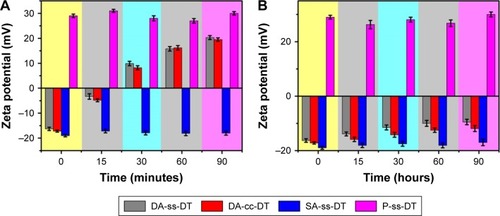
Figure 4 Cellular uptake of nanoparticles at different pHs.
Notes: (A) Fluorescence microscopy images of PC-3 cells incubated with DA-ss-DT, DA-cc-DT, SA-ss-DT, and P-ss-DT at pH 6.5 and 7.4 for 4 hours (scale bar=100 µm). (B and C) Fluorescence intensity of PC-3 cells treated with DA-ss-DT, DA-cc-DT, SA-ss-DT, and P-ss-DT at pH 6.5 and 7.4 for 4 hours. ***P<0.001. Data are represented as mean±SD (n=3).
Abbreviation: DOX, doxorubicin.
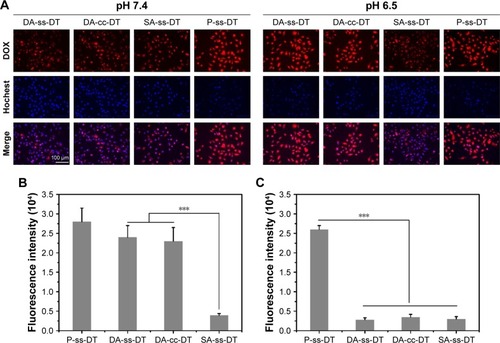
Figure 5 Size distribution of DA-ss-DT (A) and DA-cc-DT (B) in response to GSH determined by DLS measurement. Reduction-triggered release of DOX (C) and TRI (D) from DA-ss-DT and DA-cc-DT nanoparticles in PBS (pH 7.4) with 10 µM GSH. Data are represented as mean±SD (n=3).
Abbreviations: DOX, doxorubicin; DLS, dynamic light scattering; GSH, glutathione; TRI, triptolide.
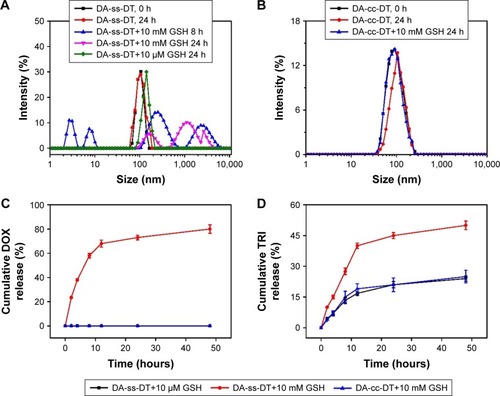
Figure 6 In vitro cytotoxicity of different drug forms to PC-3 cells under different conditions. Cell viability of PC-3 after treated with DA-ss-DT, SA-ss-DT, and P-ss-DT at pH 6.5 (A) and 7.4 (B). (C) Cell viability of DA-ss-DT and DA-cc-DT incubated with 1 mM GSH to PC-3 cells for 48 hours. All data are represented as mean±SD (n=6). **P<0.01.
Abbreviations: DOX, doxorubicin; GSH, glutathione.
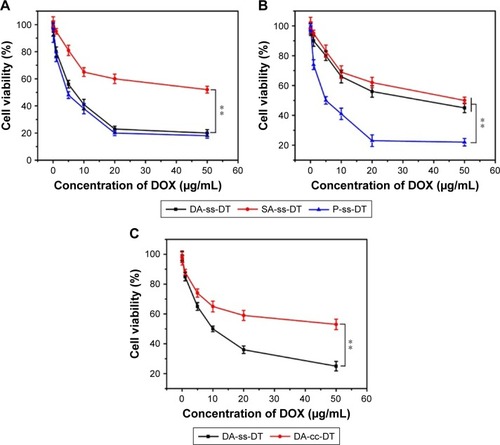
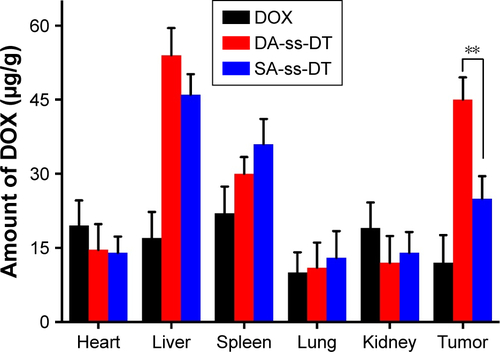
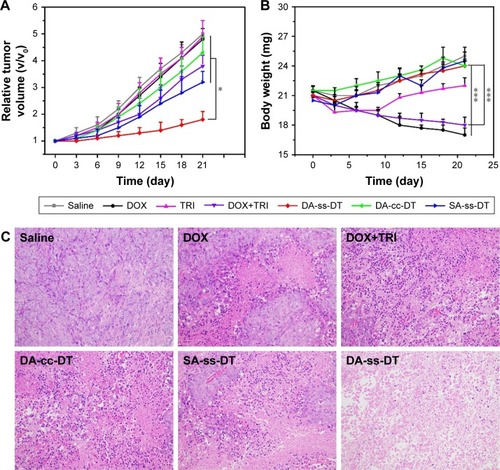
Figure 7 In vivo antitumor effects of DOX, TRI, DOX+TRI, DA-ss-DT, DA-cc-DT, and SA-ss-DT. Relative tumor volume (A) and body weight (B) of PC-3-bearing nude mice after treated with different drug forms. (C) Tumor tissue sections (stained by H&E) of mice from different groups. The sale bar is 50 µm for all images. *P<0.05 and ***P<0.001. Data are represented as mean±SD (n=6).
Abbreviations: DOX, doxorubicin; TRI, triptolide.
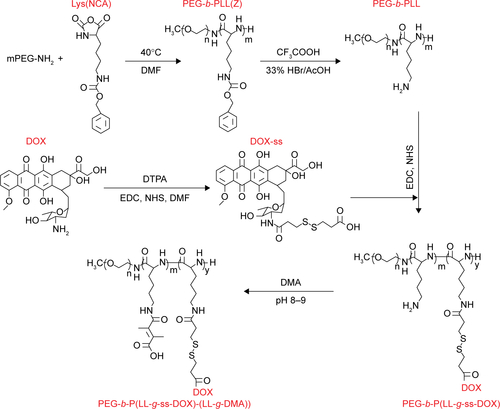
Scheme 1 Molecular structures of PEG-b-P((LL-g-ss-DOX)-(LL-g-DMA)) and schematic illustration of the multifunctional nanoparticles platform (DA-ss-DT) for in vivo DOX and TRI codelivery and therapy. The polymers and TRI can coassemble to form stable nanoparticles under nature conditions.
Notes: After intravenous administration to mice, the DA-ss-DT can extravasate from leaky tumor vasculature, and the surface charge of DA-ss-DT can change from negative to positive, resulting in quick cellular uptake of cancer cells. After internalization, the redox-sensitive DA-ss-DT nanoparticles disassembled quickly and released the two drugs, thus resulting in efficient tumor inhibited.
Abbreviations: DMA, 2,3-dimethylmaleic anhydride; DOX, doxorubicin; GSH, glutathione; TRI, triptolide.
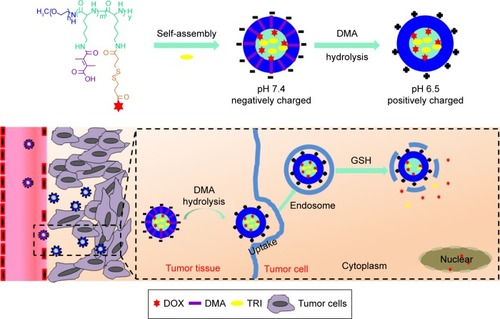
Figure S1 1H NMR spectra of DOX (A) and DTPA-DOX (B) in DMSO-d6.
Abbreviations: DMSO-d6, deuterated dimethyl sulfoxide; DOX, doxorubicin; DTPA, 3,3′-dithiodipropionic acid; NMR, nuclear magnetic resonance.
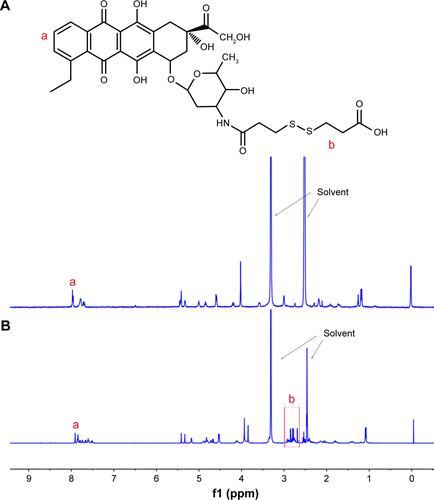
Figure S2 1H NMR spectra of PEG-b-PLLZ in DMSO-d6 (A) and PEG-b-PLL (B) in D2O.
Abbreviations: DMSO-d6, deuterated dimethyl sulfoxide; NMR, nuclear magnetic resonance; PEG-b-PLL, poly(ethylene glycol)-b-poly(L-lysine).
Figure S3 Viability of PC-3 cells incubated with DOX+TRI with different mass ratios of DOX to TRI for 48 hours at the drug concentration of 1 µg/mL.
Abbreviations: DOX, doxorubicin; TRI, triptolide.
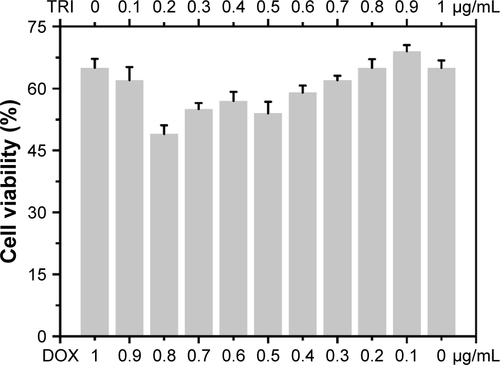
Figure S4 BSA adsorption of DA-ss-DT, DA-cc-DT, SA-ss-DT, and P-ss-DT at pH 7.4 or pH 6.5; data are represented as mean±SD (n=3).
Abbreviation: PEG-b-PLL, poly(ethylene glycol)-b-poly(L-lysine).
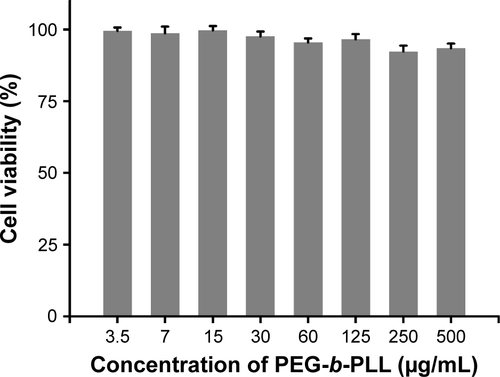
Figure S5 MTT assay of PEG-b-PLL in PC-3 cells after incubation for 48 h. Data showed mean±SD, n=6.
Abbreviation: PEG-b-PLL, poly(ethylene glycol)-b-poly(L-lysine).