Figures & data
Figure 1 The flowchart of the preparation process of NMP-NLC.
Abbreviation: NMP-NLC, nimodipine-loaded nanostructured lipid carrier.
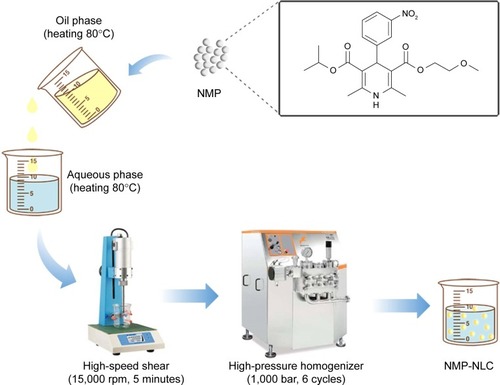
Table 1 Influence of different solid lipids on particle size, PDI, zeta potential, and EE%
Table 2 Apparent solubility of NMP in various liquid lipids
Figure 2 Formulation screening of nanostructured lipid carriers.
Notes: (A) Different ratios of solid lipid and liquid lipid (g/g) on the particle size and EE%. (B) Various ratios of drug and lipid (g/g) on the particle size and EE%. (C) Different surfactants on the particle size and EE%. (D) Different Tween 80 content (g) on particle size and EE%. Results are represented as mean ± SD (n=3).
Abbreviations: EE%, entrapment efficiency; HS 15, polyoxyethylene esters of 12-hydroxystearic acid; RH 40, hydrogenated polyoxyethylene castor oil; TPGS, vitamin E polyethylene glycol succinate.
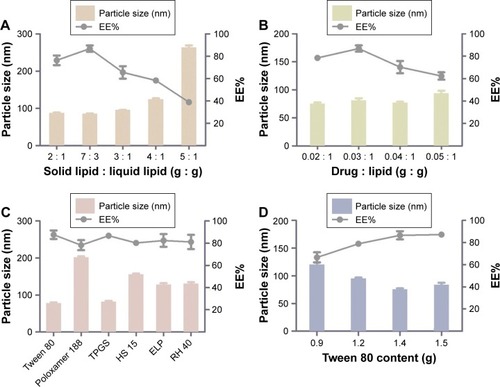
Figure 3 Technology screening of nanostructured lipid carriers.
Notes: (A) Different shear rates (×103 rpm) on the particle size and PDI. (B) Different homogenization pressures (MPa) on the particle size and PDI. (C) Different homogenization cycles on the particle size. Results were expressed as mean ± SD (n=3).
Abbreviation: PDI, polydispersity index.
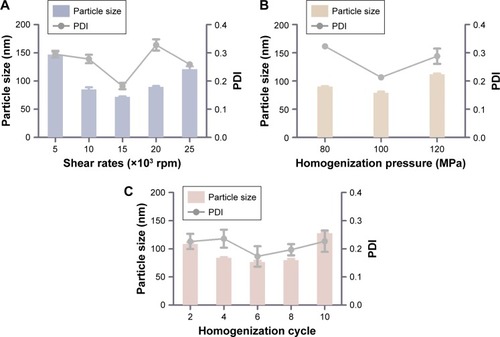
Figure 4 Pharmaceutical characteristics of NMP-NLC in vitro.
Notes: (A) Particle size and PDI of NMP-NLC analyzed by DLS. (B) TEM morphology of NMP-NLC. Bar =50 nm. (C) In vitro drug release profile of NMP-NLC and NMP suspension in pH 1.2 simulated succus gastricus. (D) In vitro drug release profile of NMP-NLC and NMP suspension in pH 6.8 simulated intestinal fluid. (E) Particle size and PDI of NMP-NLC during 2 weeks of storage at 4°C and (F) particle size and PDI of NMP-NLC during 2 weeks of storage at 25°C. Results are expressed as the mean ± SD (n=3).
Abbreviations: NMP-NLC, nimodipine-loaded nanostructured lipid carriers; PDI, polydispersity index.
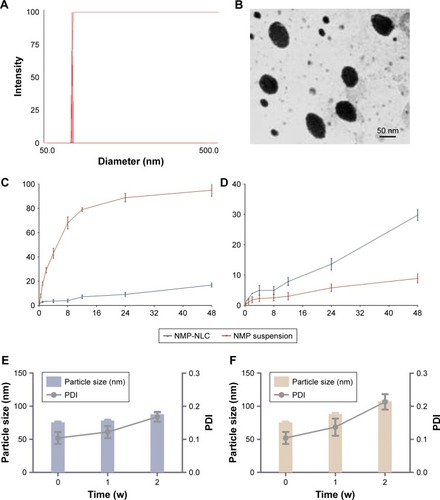
Figure 5 The crystal form of NMP-NLC.
Notes: (A) DSC thermograms of NMP coarse powder, physical mixture of lipids, and NMP and lyophilized NMP-NLC powder. (B) X-ray diffraction patterns of NMP coarse powder, physical mixture of lipids, and NMP and lyophilized NMP-NLC powder.
Abbreviations: DSC, dynamic light scattering; NMP-NLC, nimodipine-loaded nanostructured lipid carriers.
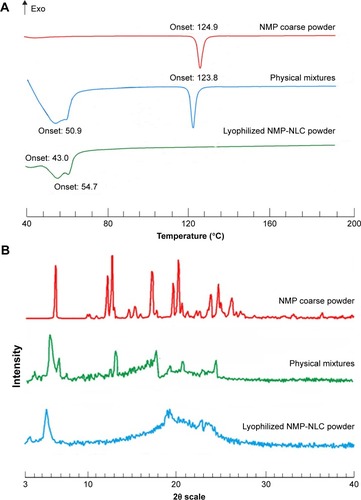
Figure 6 The FT-IR spectroscopy analysis of NMP coarse powder, physical mixture of lipids and NMP and lyophilized NMP-NLC powder.
Abbreviations: FT-IR, Fourier transform infrared spectroscopy; NMP-NLC, nimodipine-loaded nanostructured lipid carriers.
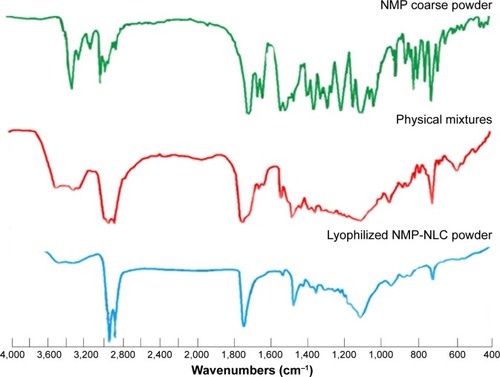
Table 3 Absorption parameters of NMP solution and NMP-NLC in different intestinal segments
Figure 7 The in situ absorption of NMP-NLC in rat intestinal segments compared with NMP solution.
Notes: (A) The absorption rate (Ka) and (B) the effective permeability coefficient (Papp). Results were represented as mean ± SD (n=3). *P<0.05, compared to the corresponding parameters of NMP solution.
Abbreviation: NMP-NLC, nimodipine-loaded nanostructured lipid carriers.
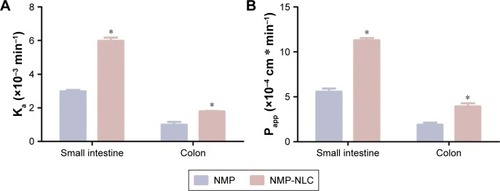
Table 4 Pharmacokinetics parameters
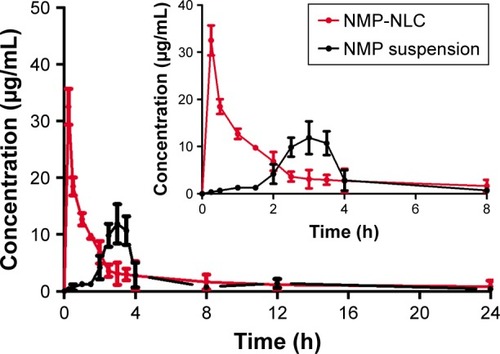
Figure 8 Plasma concentration–time curves of the pharmacokinetic experiment.
Notes: Rats were given a single oral administration at a dose of 40000 µg/kg of NMP-NLC and NMP suspension. Results are expressed as the mean ± SD (n=7).
Abbreviation: NMP-NLC, nimodipine-loaded nanostructured lipid carriers.