Figures & data
Figure 1 TEM images and digital camera photographs of protonated pentatitanate (H2Ti5O11 · H2O) (A and E), titanate after Ag+ cations exchange (B and F), partial reduction of Ag+ cations from the layered space with the UV light irradiation (C and G), and reduction of Ag+ cations with NaBH4 agent (D and H).
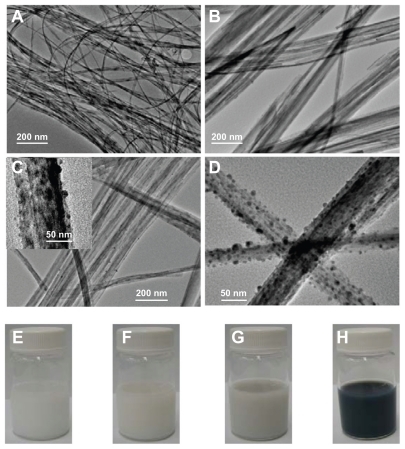
Figure 2 High resolution TEM image of Ag/titanate nanocomposite formed by UV light irradiation (300 W Xe, 320–400 nm).
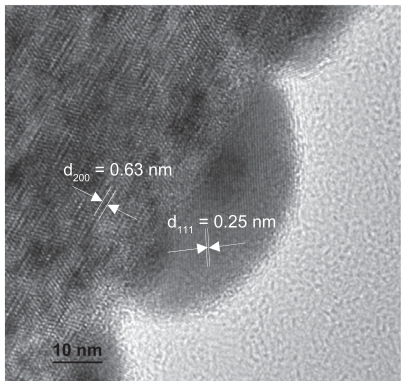
Figure 3 X-ray photoelectron spectroscopy spectra of Ag 3d: (A) titanate after Ag+ cation exchange, (B) partial reduction of Ag+ cations from the layered space with UV light irradiation, and (C) reduction of Ag+ cations with NaBH4 agent.
Abbreviation: au, arbitrary units.
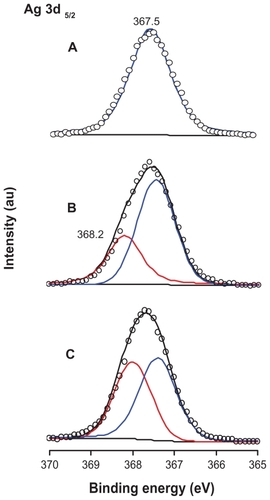
Figure 4 UV-visible absorption spectra of (A) protonated pentatitanate (H2Ti5O11 · H2O), (B) titanate after Ag+ cations exchange, (C) partial reduction of Ag+ cations from the layered space with the UV light irradiation, and (D) reduction of Ag+ cations with NaBH4 agent.
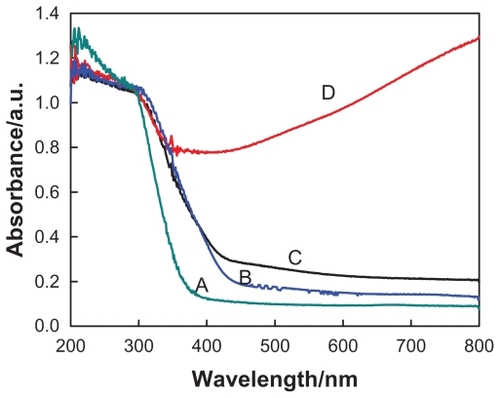
Figure 5 Comparison of the inhibition zone test for the titanate-based compounds: Ag/titanate by NaBH4 reduction (A), Ag/titanate by UV light irradiation (B), Ag2Ti5O11 · xH2O (C), and pure H2Ti5O11 · H2O (D).
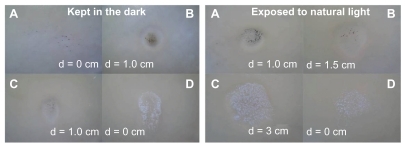
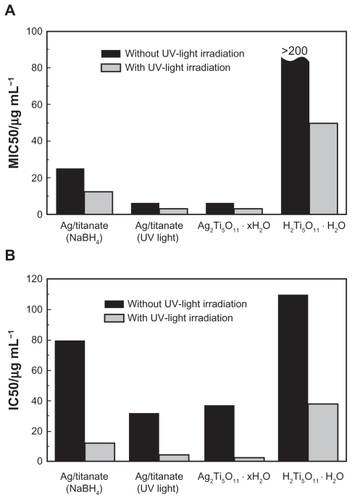
Figure 6 Antifungal (A) and antiproliferative (B) activities of titanate-based compounds, which are Ag/titanate by NaBH4 reduction, Ag/titanate by UV light irradiation, Ag2Ti5O11 · xH2O, and pure H2Ti5O11 · H2O.
Scheme 1 Schematic representations of “nutrients from galleries” principle to generate Ag/titanate nanocomposites: (A) represents the pristine protonated pentatitanate (H2Ti5O11 · H2O); (B) silver titanate (Ag2Ti5O11 · xH2O) prepared by exchange of protons with Ag+ cations; partial (C) or fully (D) Ag+ cations exchange with protons in the layered space and the formation of the silver nanoparticles on titanate substrates with the redox process. The unit cell (a–d; enclosed by dotted lines) are C-based centered monoclinic, which is projected along [010] direction.
Note: Open and filled circles indicate the locations at y = 0 and ½, respectively.
![Scheme 1 Schematic representations of “nutrients from galleries” principle to generate Ag/titanate nanocomposites: (A) represents the pristine protonated pentatitanate (H2Ti5O11 · H2O); (B) silver titanate (Ag2Ti5O11 · xH2O) prepared by exchange of protons with Ag+ cations; partial (C) or fully (D) Ag+ cations exchange with protons in the layered space and the formation of the silver nanoparticles on titanate substrates with the redox process. The unit cell (a–d; enclosed by dotted lines) are C-based centered monoclinic, which is projected along [010] direction.Note: Open and filled circles indicate the locations at y = 0 and ½, respectively.](/cms/asset/34bbe6f6-1b5a-4b06-a236-39eeb6369fb1/dijn_a_18765_f0007_c.jpg)