Figures & data
Table 1 Application of Slp-coated liposomes
Figure 1 Schematic drawing of self-reassembly of Slp on solid supports, in suspension, at an air–water interface, and on liposomes as well as in solution (nanotubes, ribbons, mono- and double-layer sheets).
Notes: Reproduced with permission from Pum D, Sleytr UB. Reassembly of S-layer proteins. Nanotechnology. 2014;25(31):312001. Available from: https://www.ncbi.nlm.nih.gov/pubmed/25030207.Citation4 © IOP Publishing. Reproduced with permission. All rights reserved.
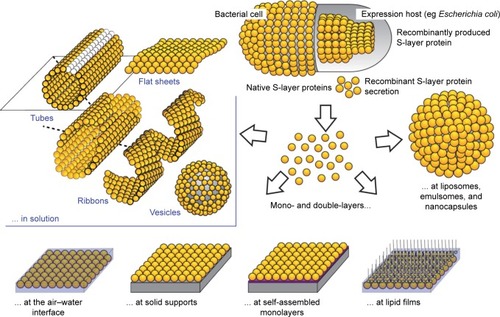
Figure 2 Schematic drawing of various Slp lattice morphologies containing oblique (p1, p2), square (p4), or hexagonal (p3, p6) symmetries.
Notes: Reproduced from Sleytr UB, Schuster B, Egelseer EM, Pum D. S-layers: principles and applications. FEMS Microbiol Rev. 2014;38(5):823–864. Creative Commons license and disclaimer available from: http://creativecommons.org/licenses/by/4.0/legalcode.Citation6
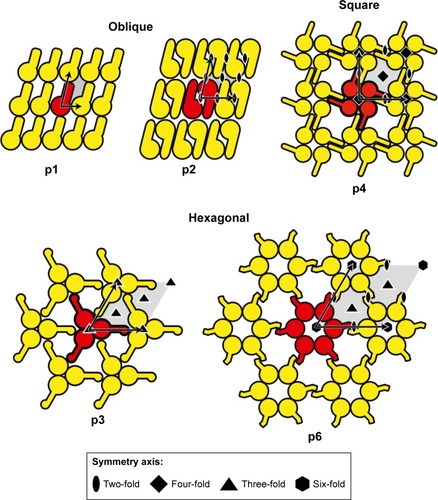
Figure 3 Brief summary of surface engineering of liposomes.
Abbreviations: PEG, polyethylene glycol; PLL, poly (L-lysine); STR-R8, stearyl octaarginine.
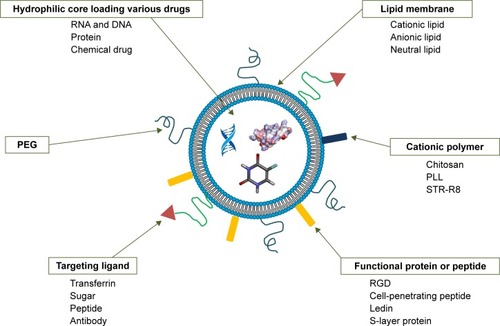
Figure 4 Schematic illustration of the five modes of interaction between Slp and the bacterial/archaeal cell envelope.
Notes: (A) In most archaea, hydrophobic transmembrane domains transfixing the cell envelope make Slp anchor at the cell envelope, and (B) in some other archaea, the lipid-modified domains in Slp drive this process. (C) Only a few archaea possess a rigid wall layer mediating the process of Slp binding to the cell envelope. (D) In Gram-positive bacteria, Slp interacts with the peptidoglycan layer indirectly via secondary cell wall polymers, (E) while Gram-negative bacterial cell envelopes express more complex components, and Slp needs to attach to the lipopolysaccharide of the outermost layer. Reproduced from Sleytr UB, Schuster B, Egelseer EM, Pum D. S-layers: principles and applications. FEMS Microbiol Rev. 2014;38(5):823–864. Creative Commons license and disclaimer available from: http://creativecommons.org/licenses/by/4.0/legalcode.Citation6
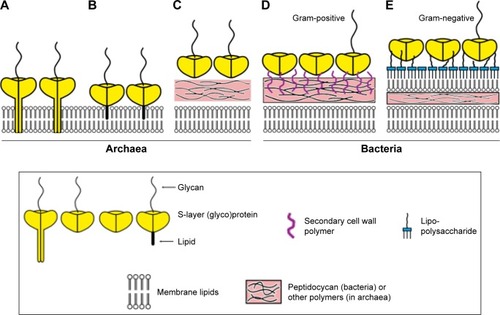
Figure 5 Schematic drawing of Slp interacting with liposomes.
Notes: The self-assembly of Slp on the surface of liposomes is mainly attributed to electrostatic forces. Intriguingly, the orientation of polar head groups of surfactant molecules tilts toward the surface normal to increase the positive charge density within linking regions to enhance the electrostatic force.
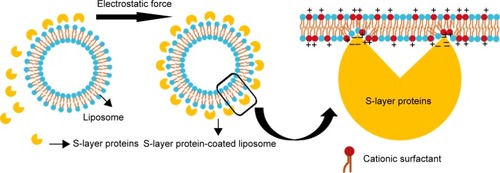
Figure 6 Schematic illustration showing the influence of cations on the formation of Slp lattices.
Notes: (A) The addition of Mg2+ leads to a change in conformation and then formation of a dynamic equilibrium between the two conformations, but the self-assembly sites are buried in the core of these two conformations, which inhibits the formation of lattices. (B) These self-assembly sites are exposed in the presence of Ca2+, (C) which facilitates the formation of lattices. Without any addition of cations, (D) the dynamic equilibrium of the three conformations provides an opportunity to expose self-assembly sites, (E) allowing lattice formation. (F) Electron microscopy observation of p4 arrays of SlpB53. Adapted by permission from Springer Nature: Springer, Eur Biophys J. Analysis of self-assembly of S-layer protein slp-B53 from Lysinibacillus sphaericus, Liu J, Falke S, Drobot B, et al. Copyright 2017.Citation42
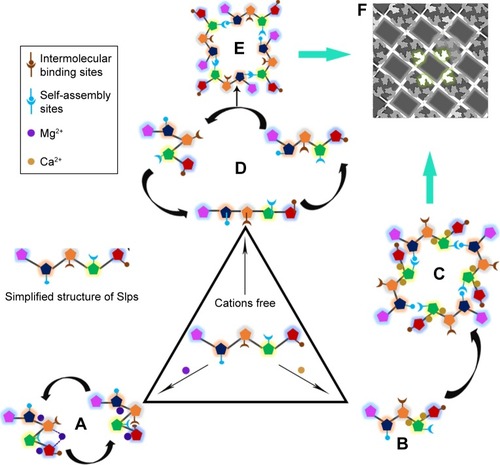
Figure 7 Evidence of the enhanced stabilities of Slp-coated liposomes.
Notes: (A) The arrays of plain liposome membrane molecules and (B) the arrays of Slp-coated liposome membrane molecules. Percentage of enclosed CF of plain liposomes and Slp-coated liposomes after incubation in (a) pancreatic extract and (b) bile salts at 37°C for 60 and 120 minutes, respectively. (C) Percentage of CF enclosed by plain liposomes and Slp-coated liposomes in an environment with pH values of 2.5, 4, and 7. (D) The release of CF from Slp-coated liposomes and plain liposomes was measured as a function of the applied temperature profile consisting of an initial isothermal phase at 25°C (0–10 minutes), followed by two cycles of heating from 25°C to 55°C (10–30 minutes; 50–70 minutes) and cooling to 25°C (30–50 minutes; 70–90 minutes). The release of CF from Slp-coated liposomes and plain liposomes was measured during (E) stirring at 3,000 rpm and 22°C and (F) sonication at 26°C. (α) Plain liposomes are recognized by opsonin, and the clearance function of the RES is activated, causing plain liposomes to be taken up by the phagocyte system. (β) Opsonin is unable to recognize Slp-coated liposomes due to blocking of the S-layer, leading to RES remaining off. (A–C) Reprinted from Biochimica et Biophysica Acta (BBA) – Biomembranes, 1768(3), Hollmann A, Delfederico L, Glikmann G, de Antoni G, Semorile L, Disalvo EA, Characterization of liposomes coated with S-layer pro teins from lactobacilli, 393–400, Copyright (2007), with permission from Elsevier (D–F) Reprinted from Biochimica et Biophysica Acta (BBA) – Biomembranes, 1418(1), Mader C, Küpcü S, Sára M, Sleytr UB, Stabilizing effect of an S-layer on liposomes towards thermal or mechanical stress, 106–116, Copyright (1999), with permission from Elsevier.
Abbreviations: CF, carboxyfluorescein; RES, reticuloendothelial system.
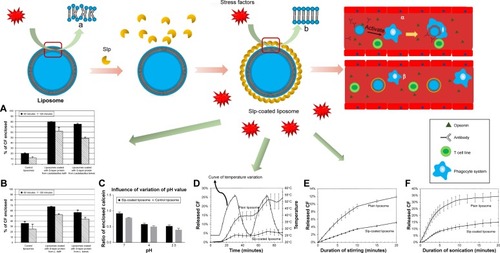
Figure 8 Mechanism of Slp promoting drug internalization of liposomes and fluorescence microscopy of gastric and intestinal mucosal scrapings after (A) 7 and (B) 12 hours of intragastric administration of Slp-coated liposomes and plain liposomes, respectively.
Notes: (a) Gastric mucosa of Slp-coated liposomes. (b) Gastric mucosa of plain liposomes. (c) Intestinal mucosa of Slp-coated liposomes. (d) Intestinal mucosa of plain liposomes. Fluorescent images are Reprinted from Int J Pharm, 529(1–2), Wang W, Shao A, Feng S, Ding M, Luo G, Physicochemical char acterization and gastrointestinal adhesion of S-layer proteins-coating liposomes, 227–237, Copyright (2017), with permission from Elsevier.
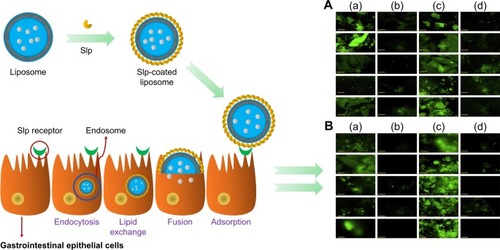
Table 2 Summary of Slps with their receptors and their targeted cells or locations
Table 3 Summary of biomacromolecules immobilized by Slp and Slfp
Figure 9 Schematic drawing and TEM images of (A, A1, and A2) rSbpA-EGFP-coated liposomes, (B, B1, B2, and B3) IgG-rSbpA-GG-coated emulsomes, (C and C1) ferritin-rSlp-streptavidin fusion protein-coated liposomes, and (D and D1) ferritin-streptavidin-biotin bridge-Slp-coated liposomes.
Notes: TEM images were: (A1 and A2) Copyright ©2004. Portland Press, Ltd. Reproduced from Ilk N, Küpcü S, Moncayo G, et al. A functional chimaeric S-layer-enhanced green fluorescent protein to follow the uptake of S-layer-coated liposomes into eukaryotic cells. Biochem J. 2004;379(Pt 2):441–448.Citation111 (B1–B3) Reproduced from Ücisik MH, Küpcü S, Breitwieser A, Gelbmann N, Schuster B, Sleytr UB. S-layer fusion protein as a tool functionalizing emulsomes and CurcuEmulsomes for antibody binding and targeting. Colloids Surf B Biointerfaces. 2015;128:132–139. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4406452/. Creative Commons license and disclaimer available from: http://creativecommons.org/licenses/by/4.0/legalcode.Citation137 (C1) Adapted with permission from Moll D, Huber C, Schlegel B, Pum D, Sleytr UB, Sara M. S-layer-streptavidin fusion proteins as template for nanopatterned molecular arrays. Proc Natl Acad Sci. 2002;99(23):14646–14651. Copyright (2002) National Academy of Sciences, U.S.A.Citation67 (D1) Reprinted from Biochim ica et Biophysica Acta (BBA) – Biomembranes, 1463(1), Mader C, Küpcü S, Sleytr UB, Sára M, S-layer-coated liposomes as a versatile system for entrapping and binding target molecules, 142–150. Copyright (2000), with permission from Elsevier.
Abbreviation: TEM, transmission electron microscopy.
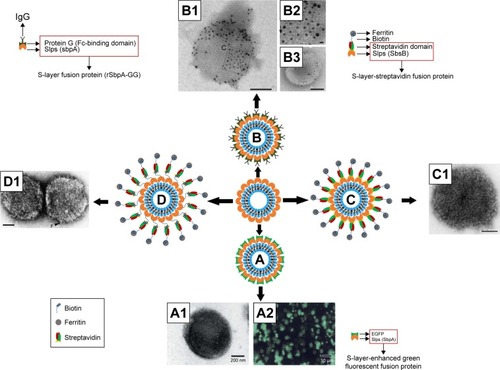
Figure 10 Evidence of Slp-coated liposomes promoting drug internalization and surface engineering of liposomes and as carriers of vaccines for oral administration.
Notes: Flow cytometry histograms of Caco-2 cells before (red line) and after 30 minutes of (green line) incubation at 37°C with (A) control and (B) Slp-coated liposomes. (C) Calcein dequenching of Caco-2 cells incubated with Slp-coated liposomes and plain liposomes at 37°C or 4°C; flow cytometry histograms of Caco-2 cells upon 30-minute incubation at 4°C (blue line) and 37°C (green line) with (D) control and (E) S-layer coated liposomes; (F) viability of Caco-2 cells incubated with Slp-coated liposomes and control liposomes; (G) IgG concentration induced by oral delivery of Slp-coated liposomes carrying hepatitis B vaccine. (H) Schematic drawing of rSbpA-GG facilitating emulsomal surface immobilization of IgG. (I) Confocal microscopy observing the interaction of HIgG-FITC (green fluorescence) with rSbpA-GG-coated emulsome-entrapped Sudan β (red fluorescence). (A–F) Reproduced from Hollmann A, Delfederico L, Santos NC, Disalvo EA, Semorile L. Interaction of S-layer proteins of Lactobacillus kefir with model membranes and cells. J Liposome Res. 2018;28(2):117–125 by permission of the publisher (Taylor & Francis Ltd, http://www.tandfonline.com).Citation98 (G) Reproduced with permission from Shao.Citation139 Copyright 2017, Zhejiang University of Technology. (H and I) Reproduced from Ücisik MH, Küpcü S, Breitwieser A, Gelbmann N, Schuster B, Sleytr UB. S-layer fusion protein as a tool functionalizing emulsomes and CurcuEmulsomes for antibody binding and targeting. Colloids Surf B Biointerfaces. 2015;128:132–139. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4406452/. Creative Commons license and disclaimer available from: http://creativecommons.org/licenses/by/4.0/legalcode.Citation137 *P<0.05; ***P<0.001.
Abbreviations: FITC, fluorescein isothiocyanate; HIgG, human IgG; ns, not significant.
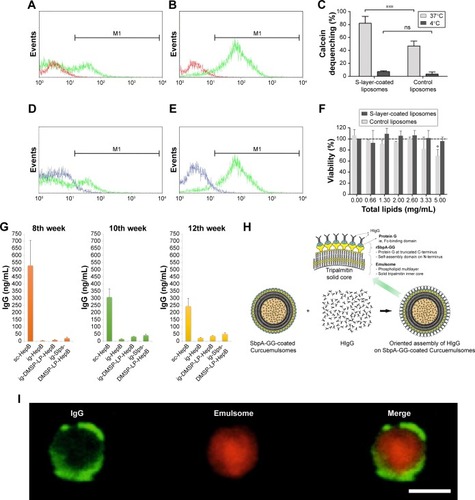
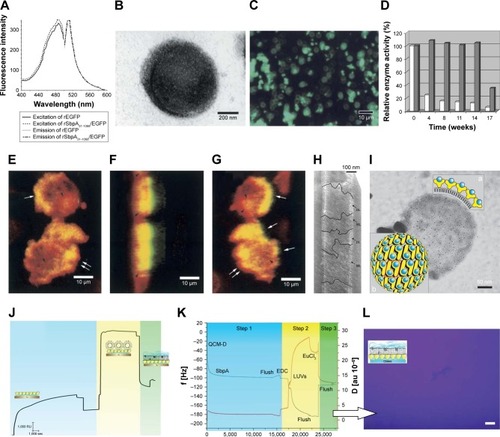
Figure 11 Biomedical applications of Slp-coated liposomes in imaging technology, as biocatalysts, and in preparation of biomimetic membranes.
Notes: (A) Comparison of fluorescence excitation and emission spectra of rEGFP and rSbpA31-1068/EGFP. (B) TEM image of rSbpA31-1068/EGFP-coated liposomes. (C) Fluorescence microscopy observation of rSbpA31-1068/EGFP-coated liposomes. (D) Time-dependent enzyme activity of rSgsE/RmlA and sole RmlA. (E) Two-dimensional confocal microscopy image of the interaction between HeLa cells and rSbpA31-1068/EGFP-coated liposomes; three-dimensional confocal microscopy observation of the interaction between HeLa cells and rSbpA31-1068/EGFP-coated liposomes based on a confocal z-stack with the view at an anticlockwise rotation around the y-axis by (F) 96° and (G) 162° relative to the focal plane. (H) Electron micrograph of rSgsE/RmlA self-assembling nanotube biocatalysts. (I) Electron micrograph of rSgsE/RmlA-coated liposomal biocatalysts. (a) schematic cross section of a biocatalytic liposome, showing the surface display of the RmlA epitopes within the nanolattice; (b) schematic representation of the p2 symmetry of the G_SgsE.RmlA nanolattice. Blue circles:biocatalytic RmlA epitopes (arbitrary positions); yellow symbols: rSgsE. (J) SPR spectroscopy and (K) QCM-D characterization of the formation process of planar biomimetic membrane. (L) Fluorescence microscopy confirming the formation of planar biomimetic membranes. (A–C) and (E–G) Copyright ©2004. Portland Press, Ltd. Reproduced from Ilk N, Küpcü S, Moncayo G, et al. A functional chimaeric S-layer-enhanced green fluorescent protein to follow the uptake of S-layer-coated liposomes into eukaryotic cells. Biochem J. 2004;379(Pt 2):441–448.Citation111 (D, H, and I) Copyright ©2007. John Wiley and Sons. Reproduced from Schäffer C, Novotny R, Küpcü S, et al. Novel biocatalysts based on S-layer self-assembly of Geobacillus stearothermophilus NRS 2004/3a: a nanobiotechnological approach. Small. 2007;3(9):1549–1559.Citation148 (J–L) Reproduced with permission from IOP Publishing, Schrems A, Larisch VD, Stanetty C, et al, Liposome fusion on proteinaceous S-layer lattices triggered viaβ-diketone ligand–europium(iii) complex formation, Soft Matter. 2011;7(12):5514–5518. First published on May 24, 2011. © IOP Publishing. Reproduced with permission. All rights reserved.
Abbreviations: QCM-D, quartz crystal microbalance with dissipation monitoring; SPR, surface plasmon resonance; TEM, transmission electron microscopy.