Figures & data
Table 1 Primers used for real-time PCR
Table 2 Elemental composition at the surface of various Ti discs with different treatments as determined by XPS
Figure 1 Flow diagram of the experimental procedure.
Abbreviations: ACS, ASA-loaded chitosan nanoparticles; ASA, aspirin; BMSCs, bone marrow stromal cells; CM, conditioned medium; COL, collagen; HA, sodium hyaluronate; LPS, lipopolysaccharides; PTL, phase-transited lysozyme; qPCR, quantitative PCR; SEM, scanning electron microscopy; TEM, transmission electron microscopy; Ti, titanium; VG, Van Gieson; XPS, X-ray photoelectron spectroscopy.
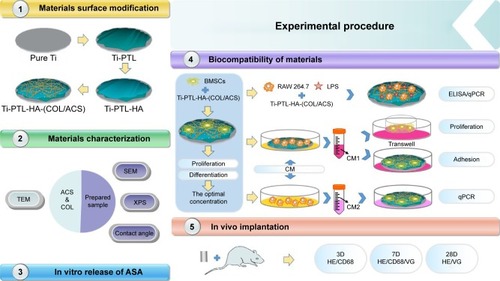
Figure 2 Surface characteristics.
Notes: (A) TEM characterization of (a) self-assembled COL I, (b) ACS dispersed in collagen solution; the black arrow indicates the ACS, and the white arrow indicate collagen fibrils. (B) FE-SEM surface morphologies of (a) pure Ti, (b) Ti-PTL, (c) Ti-PTL-HA, (d) Ti-PTL-HA-(COL/ACS). (C) Contact angle of SBF on the surface of the tested samples (n=3). *P<0.05, compared with pure Ti. (D) XPS wide-scan spectra of samples after having been immersed in deionized water for 7 days.
Abbreviations: ACS, aspirin-loaded chitosan nanoparticles; COL I, type I collagen; FE-SEM, field-emission scanning electron microscopy; HA, sodium hyaluronate; PTL, phase-transited lysozyme; SBF, simulated body fluid; TEM, transmission electron microscopy; Ti, titanium; XPS, X-ray photoelectron spectroscopy.
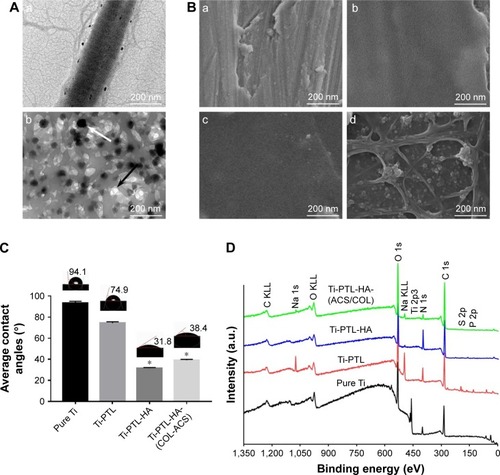
Figure 3 (A) Release curve of different volumes of ASA in the preparation process from multilayers at 1, 2, 3, 4, 5, 6, and 7 days (n=3). (B) CCK-8 results of BMSCs after culturing for 1, 3, and 5 days on the different surfaces of Ti discs (n=3). (C) ALP activities of BMSCs cultured for 7 and 14 days on different surfaces of Ti discs (n=3).
Notes: *denotes the significant difference between Ti and other groups; #denotes the significant difference between different Ti and other groups; &denotes the significant difference between different ASA microspheres: *, #, & (P<0.05); && (P<0.01).
Abbreviations: ASA, aspirin; BMSCs, bone marrow stromal cells; CCK-8, Cell Counting Kit-8; COL, collagen; Ti, titanium.
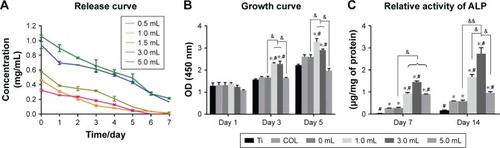
Figure 4 Verifying the success of LPS induction by ELISA and the effect of specimen-conditioned medium of RAW264.7 cells on proinflammatory cytokine expression on Ti surfaces as detected by ELISA and qPCR.
Notes: (A) (a) NaNO2 content, (b) TNF-α content; (B) (a) NaNO2 content, (b) TNF-α gene, and (c) IL-6 gene. (*P<0.05, n=3).
Abbreviations: ASA, aspirin; IL-6, interleukin-6; LPS, lipopolysaccharides; NaNO2, nitrite; qPCR, quantitative PCR; TNF-α, tumor necrosis factor-α.
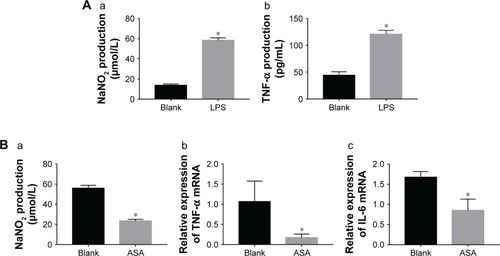
Figure 5 Adhesion, proliferation, migration, and osteogenic differentiation of BMSCs in conditioned medium.
Notes: (A) Adhesion of BMSCs on different Ti surfaces; (B) migration of BMSCs on Ti surfaces in Transwell: greater BMSC migration was observed on the ASA-loaded Ti surface than on the blank surface; (C) effects of the conditioned medium of RAW264.7 cells on osteogenic-related gene expression of BMSCs after culturing for 7 and 14 days, as detected by qPCR: (a) COL I gene, (b) OCN gene, (c) ALP gene, and (d) RUNX2 gene. *P<0.05, n=3.
Abbreviations: ASA, aspirin; BMSCs, bone marrow stromal cells; COL I, type I collagen; OCN, osteocalcin; qPCR, quantitative PCR; RUNX2, runt-related transcription factor 2; Ti, titanium.
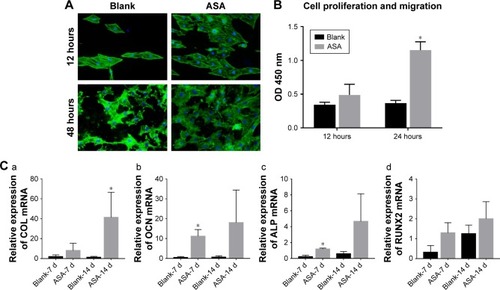
Figure 6 Histological analysis of bone formation in vivo.
Notes: Three days after implantation: the small, deeply stained cells adjacent to the surface of the implants were inflammatory cells infiltrating the tissues. In addition, multinucleated giant cells were scattered in the tissues. There was little difference between the two groups. Seven days after implantation: the inflammatory cells infiltrating the implant surface were significantly more numerous in the blank group than in the ASA group, and bone matrix began to appear in the ASA group. Twenty-eight days after implantation: short, cubic preosteoblasts arranged around the trabecular bone were well formed around the ASA-loaded implants; at the same time, newly formed, small blood vessels were scattered in the trabecular bone at the ASA-loaded surface.
Abbreviation: ASA, aspirin.
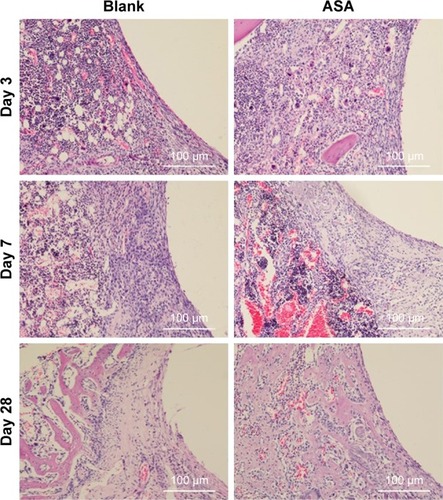
Figure 7 VG staining: representative undecalcified histological images of new bone formation around and within the implants detected by VG staining.
Notes: (A) The bone tissue is stained red, and the fibrous tissue is stained yellow and white. (B) Histomorphometric measurement of bone volume fraction within the implants showed that the ASA group has the highest amount of new bone ingrowth, which is higher than that in the blank group (*P<0.05, n=3).
Abbreviations: ASA, aspirin; BV/TV, the new bone volume fraction; VG staining, Van Gieson staining.
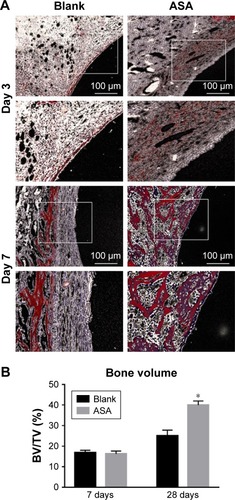
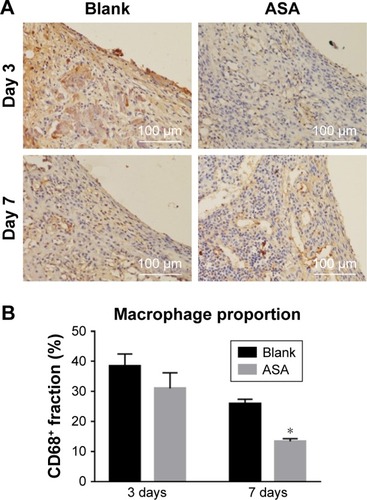
Figure 8 Immunofluorescence staining for CD68+ cells.
Notes: A brownish yellow color indicates positive macrophage infiltration. (A) The numbers of inflammatory cells around the surfaces of two groups did not differ significantly on the 3rd day. On the 7th day, fewer inflammatory cells were observed on the surfaces of the ASA group than on the surfaces of the blank group. (B) The ASA group elicited a lower proportion (%) of CD68+ macrophages than the blank group. *P<0.05, n=3.
Abbreviation: ASA, aspirin.