Figures & data
Figure 1 Characterization of MH.
Notes: (A) General morphological map of MH. (B) SEM image of MH (scale bar represents 100 µm, magnification ×500). (C) Cytotoxicity test of MH (mean ± SD, n=6). (D) The degradation of MH in vitro (mean ± SD, n=3, ***P<0.001). (E) The degradation of MH in vivo (red arrows point to the tumor tissues; gels that remained are circled).
Abbreviations: MH, MMP-responsive hydrogel; MMP, matrix metalloproteinase; SEM, scanning electron microscopy.
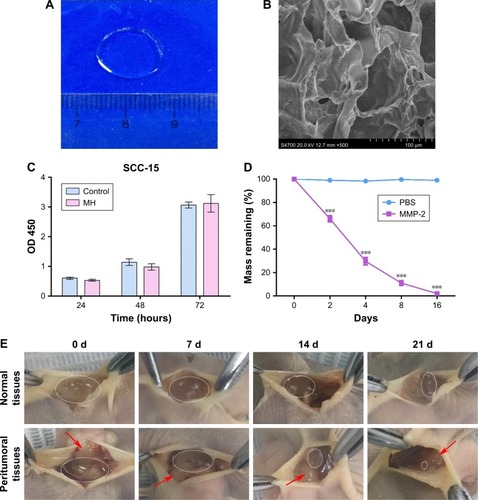
Figure 2 The morphology and characterization of NDIMH.
Notes: (A) Representative photographs of DOX and ICG nanoparticles dispersed in PBS at ambient temperature (red represents DOX nanoparticles, and green represents ICG nanoparticles). (B) TEM image of nanoparticles (scale bar represents 1 µm, magnification ×8,000). (C) General morphological map of NDIMH. (D) SEM image of NDIMH (scale bar represents 100 µm, magnification ×250). (E) The size distribution of DOX nanoparticles. (F) The size distribution of ICG nanoparticles. (G) The nano DOX release profile of NDIMH at varied time points. (H) The nano ICG release profile of NDIMH at varied time points.
Abbreviations: DOX, doxorubicin; ICG, indocyanine green; MMP, matrix metalloproteinase; NDIMH, nano DOX-ICG MMP-responsive hydrogel; SEM, scanning electron microscopy; TEM, transmission electron microscopy.
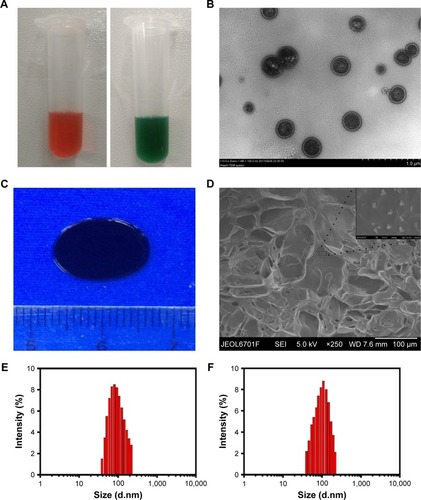
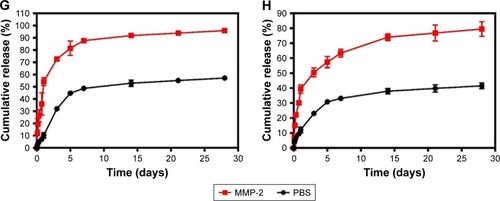
Figure 3 Evaluation of the photosensitivity properties of NDIMH.
Notes: (A) Maximum temperature profiles of PBS, free ICG, free nano ICG, NIMH, and NDIMH under NIR laser irradiation in vitro. (B) Maximum temperature profiles of the irradiated area of nude mice bearing tumors injected with PBS, free ICG, free nano ICG, NIMH, and NDIMH in vivo. (C) Infrared thermographic maps of a 24-well plate after 10 minutes of irradiation. (D) Infrared thermographic maps of mice after intratumoral injection at 6 minutes after laser irradiation. (E) The ROS generation of different formulations (scale bar represents 25 µm, magnification ×630). (F) The cellular uptake of DOX in SCC-15 (scale bar represents 25 µm, magnification ×500). (G) The quantitative analysis of ROS generation (mean ± SD, n=3). (H) The quantitative analysis of cellular uptake of DOX (mean ± SD, n=3, ***P<0.001).
Abbreviations: DOX, doxorubicin; ICG, indocyanine green; NDIMH, nano DOX-ICG matrix metalloproteinase-responsive hydrogel; NDMH, nano DOX matrix metalloproteinase-responsive hydrogel; NIMH, nano ICG matrix metalloproteinase-responsive hydrogel; NIR, near infrared.
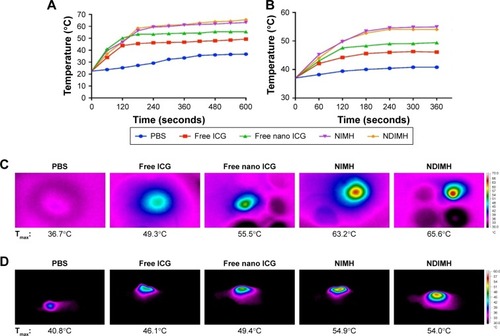
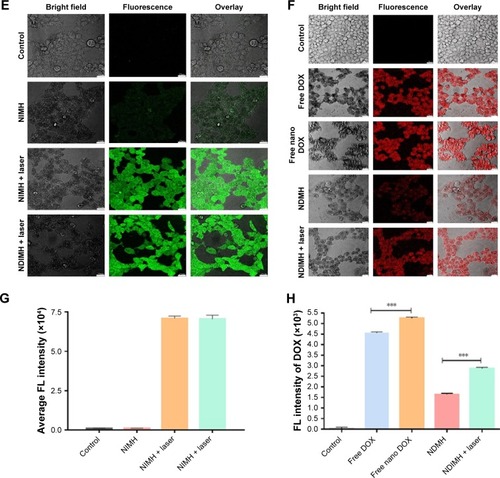
Figure 4 The functions of NDIMH in proliferation, motion, and invasion of SCC-15 tumor cells in vitro.
Notes: (A) Effects of different formulations on SCC-15 cells viability at 48 hours (mean ± SD, n=6, *P<0.05, **P<0.01, ***P<0.001). (B) Effects of NDIMH + laser treated with/without MMP-2 on SCC-15 cells motion at 24 hours (scale bar: 100 µm). (C) The quantitative analysis of wound healing assay (mean ± SD, n=3, ***P<0.001). (D) Effects of NDIMH + laser treated with/without MMP-2 on the invasion of SCC-15 cells (scale bar: 100 µm). (E) The quantitative analysis of transwell assay (mean ± SD, n=3, ***P<0.001).
Abbreviations: MH, MMP-responsive hydrogel; MMP, matrix metalloproteinase; NDIMH, nano doxorubicin-indocyanine green MMP-responsive hydrogel; NDMH, nano doxorubicin MMP-responsive hydrogel; NIMH, nano indocyanine green MMP-responsive hydrogel.
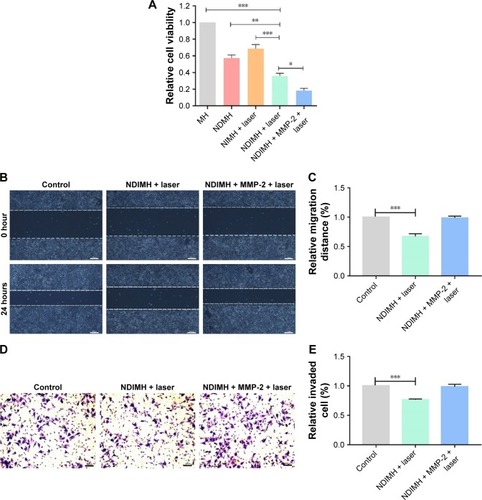
Figure 5 Antitumor effects and biosafety evaluation of NDIMH in vivo.
Notes: (A) SCC-15 tumor growth curves of different groups after intratumoral injection up to 21 days (mean ± SD, n=3, **P<0.01, ***P<0.001). (B) SCC-15 tumor mass histogram of different groups 21 days after treatments (mean ± SD, n=3, *P<0.05, **P<0.01, ***P<0.001). (C) The photograph of excised tumors 21 days after treatments. (D) The body weight changes of the mice during treatments (mean ± SD, n=3). (E) TUNEL apoptosis assay of tumor sections 21 days after treatments. Cells stained brown indicated apoptotic cells (scale bar represents 50 µm, magnification ×40). (F) Immunohistochemical staining for Ki67 expression of tumor sections 21 days after treatments. Cells stained brown indicated proliferating cells (scale bar represents 50 µm, magnification ×40). (G) Histological sections of the main organs (brain, heart, liver, spleen, lungs, and kidneys) were stained by H&E.
Abbreviations: NDIMH, nano doxorubicin-indocyanine green matrix metalloproteinase-responsive hydrogel; NDMH, nano doxorubicin matrix metalloproteinase-responsive hydrogel; NIMH, nano indocyanine green matrix metalloproteinase-responsive hydrogel; NS, normal saline.
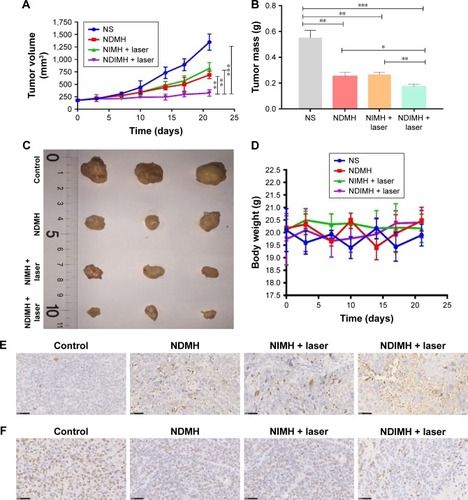
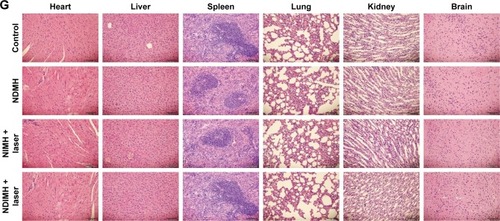
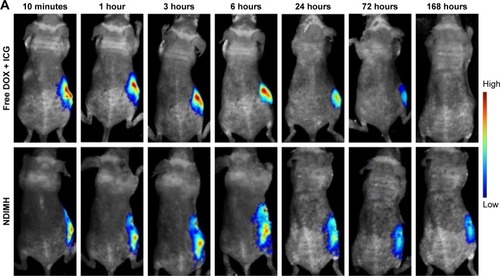
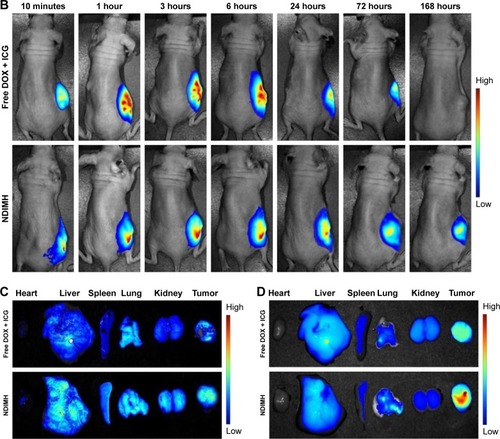
Figure 6 In vivo FL imaging and biodistribution of DOX/ICG in mice bearing SCC-15 tumors after intratumoral injection of free DOX + ICG or NDIMH.
Notes: (A) Time-lapse DOX FL of the whole body of mice. (B)Time-lapse ICG FL of the whole body of mice. (C) DOX FL intensity of major organs and tumors after 21 days of administration. (D) ICG FL intensity of major organs and tumors after 21 days of administration.
Abbreviations: DOX, doxorubicin; FL, fluorescence; ICG, indocyanine green; NDIMH, nano DOX-ICG matrix metalloproteinase-responsive hydrogel.