Figures & data
Figure 1 Illustration of a ligand-mediated active targeting.
Notes: A carrier(1) loaded with the drug (2) is treated with a ligand (3) capable of recognizing the binding positions (4) on the surface of the cell (5). Reprinted by permission from Springer Nature Customer Service Centre GmbH: Springer Nature. Passive and active drug targeting drug delivery to tumors as an example. By Torchilin VP. In: Schäfer-Korting M, editor. Drug Delivery. Handbook of Experimental Pharmacology. Berlin, Heidelberg: Springer; 2009:3–53. Copyright 2009.Citation74
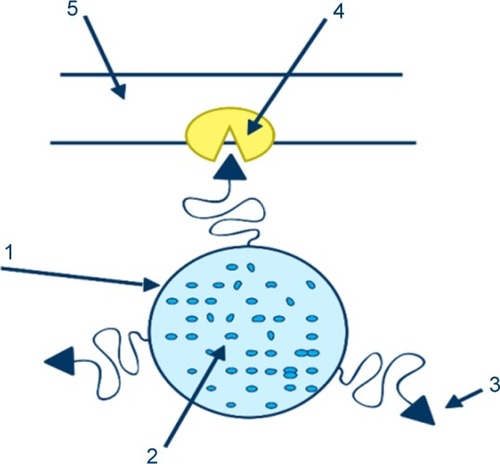
Figure 2 In passive targeting (A), drug targeting can be described as passive when nanoparticles diffused via the leaky tumor vessels. While in active targeting (B), drug delivery took place when the ligands of the nanocarriers were attached to the receptor on the tumor cells.
Notes: Reprinted by permission from Springer Nature Customer Service Centre GmbH: Springer Nature. Systemic targeting systems-EPR effect, ligand targeting systems. By Pawar PV, Domb AJ, Kumar N. In: Domb AJ, Khan W, editors. Focal Controlled Drug Delivery. Boston, MA: Springer US; 2014:61–91. Copyright 2014.Citation75
Abbreviation: NP, nanoparticle.
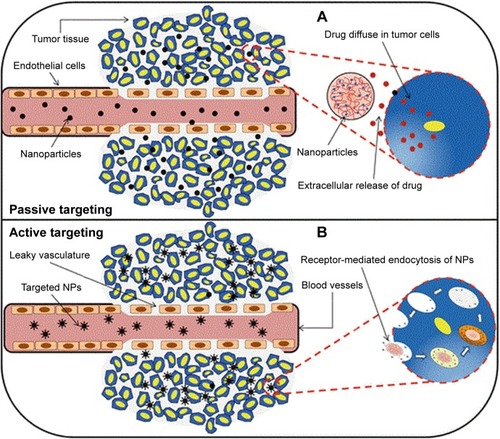
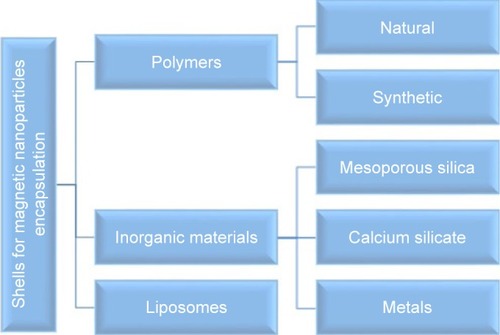
Table 1 Fundamental characteristics of a targeted drug delivery system
Figure 4 Schematic illustration describing the effective nanocombinations therapy.
Notes: Reprinted with permission from Vivek R, Thangam R, Kumar SR, et al. HER2 targeted breast cancer therapy with switchable “Off/On” multifunctional “Smart” magnetic polymer core-shell nanocomposites. ACS Appl Mater Interfaces. 2016; 8(3):2262–2279. Copyright © 2016, American Chemical Society.Citation123
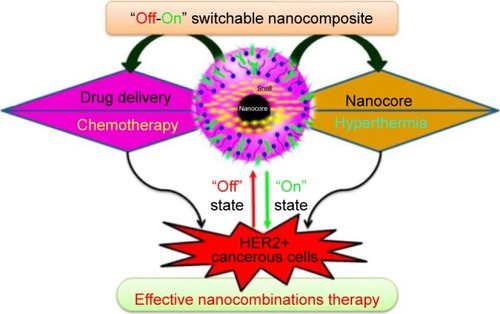
Figure 5 TEM results of (A–C) SG-1, (D–F) SG-2, and (G–I) SG-3 composites.
Notes: The composites prepared with 0.22, 0.31, and 0.45 mmol of TEOS were named as SG-1, SG-2, and SG-3 composites, respectively. Reproduced from Uribe Madrid SI, Pal U, Kang YS, Kim J, Kwon H, Kim J. Fabrica tion of Fe3O4@mSiO2 core-shell composite nanoparticles for drug delivery applications. Nanoscale Res Lett. 2015;10(1):217. Copyright © Uribe Madrid et al.; licensee Springer. 2015. Creative Commons License available from: https://creativecommons.org/licenses/by/4.0/legalcode.Citation171
Abbreviations: TEM, transmission electron microscopy; TEOS, tetraethylorthosilicate.
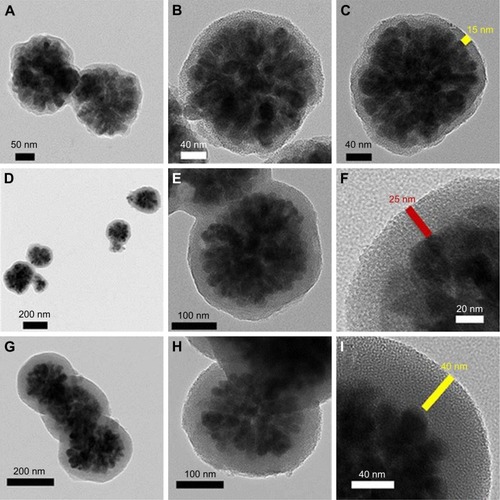
Figure 6 Ibuprofen release rate in different wall thicknesses.
Notes: Reproduced from Uribe Madrid SI, Pal U, Kang YS, Kim J, Kwon H, Kim J. Fabrica tion of Fe3O4@mSiO2 core-shell composite nanoparticles for drug delivery applications. Nanoscale Res Lett. 2015;10(1):217. Copyright © Uribe Madrid et al.; licensee Springer. 2015. Creative Commons License available from: https://creativecommons.org/licenses/by/4.0/legalcode.Citation171
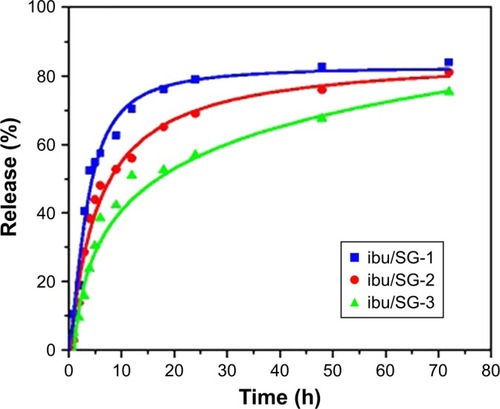
Figure 7 (A) Synthesis of magnetic iron oxide–calcium silicate mesoporous core–shell nanocomposites with a hollow structure using two liquid phase system and ultrasound irradiation in the presence of isooctane. (B, C) TEM of the nanocomposites fabricated by using two liquid phase systems and ultrasound irradiation in the presence of isooctane.
Notes: Reprinted with permission from Lu BQ, Zhu YJ, Ao HY, Qi C, Chen F. Synthesis and characterization of magnetic iron oxide/calcium silicate mesoporous nanocomposites as a promising vehicle for drug delivery. ACS Appl Mater Interfaces. 2012;4(12):6969–6974. Copyright © 2012, American Chemical Society.Citation185
Abbreviations: TEM, transmission electron microscopy; TEOS, tetraethylorthosilicate.
Figure 8 Schematic illustration of the synthesis of DLMCs.
Notes: Reprinted with permission from Cao Z, Yue X, Li X, Dai Z. Stabilized magnetic cerasomes for drug delivery. Langmuir. 2013;29(48):14976–14983. Copyright © 2013, American Chemical Society.Citation194
Abbreviations: DLMC, DOX-loaded magnetic cerasome; DOX, doxorubicin.
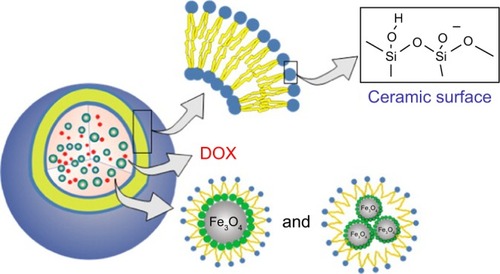
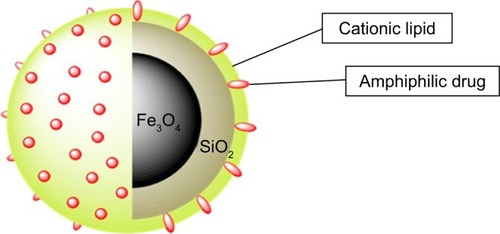
Figure 9 SLB-MNPs consisted of iron oxide–silica nanoparticles enclosed in a cationic lipid bilayer that contains the amphiphilic DOX.
Notes: Reprinted with permission from Mattingly SJ, O’Toole MG, James KT, Clark GJ, Nantz MH. Magnetic nanoparticle-supported lipid bilayers for drug delivery. Langmuir. 2015;31(11):3326–3332. Copyright © 2015, American Chemical Society.Citation195
Abbreviations: DOX, doxorubicin; MNP, magnetic nanoparticle; SLB, supported lipid bilayer.