Figures & data
Figure 1 AMX-loaded LNPs, which were designed to release AMX near H. pylori. The double-emulsion LNPs are composed of cetyl palmitate, Tween 80, linolenic acid, and DOPE.
Abbreviations: AMX, amoxicillin; DOPE, dioleoylphosphatidylethanolamine; H. pylori, Helicobacter pylori; LNPs, lipid nanoparticles.
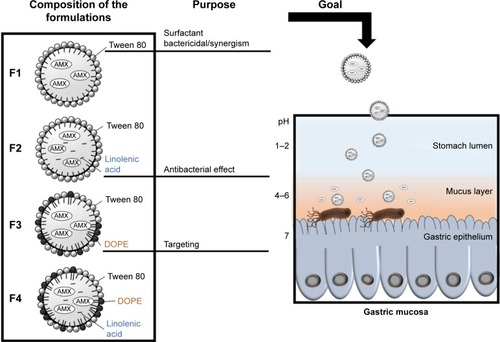
Table 1 Composition and physical characterization (size, PDI, zeta potential, and LC) of the four AMX-loaded LNPs and the corresponding unloaded LNPs
Figure 2 TEM images of the AMX-loaded LNPs and the corresponding unloaded LNPs.
Notes: P1, F1, P2, F2, F3, P4A, and F4A are at a magnification of 50,000×. P3 is at a magnification of 25,000×. P4B and F4B are at a magnification of 100,000×.
Abbreviations: AMX, amoxicillin; LNPs, lipid nanoparticles; TEM, transmission electron microscopy.
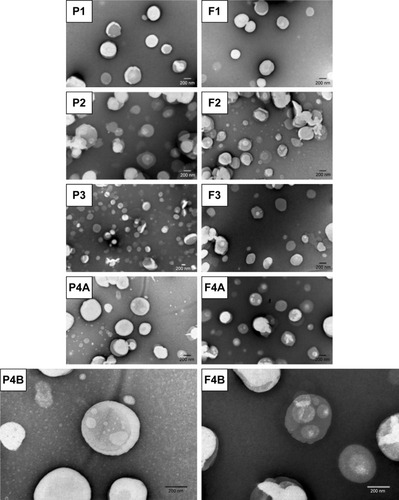
Figure 3 Characterization of the LNPs suspensions. (A) LNPs size and PDI. Bars represent the size (left y-axis) and dots represent the PDI (right y-axis). (B) LNPs zeta potential. (C) LNPs LC. The parameters were evaluated over time (0 months [dark grey], 3 months [intermediate grey] and 6 months [light grey]).
Notes: Values represent the mean±SD of three independently produced formulations. *P<0.05, **P<0.01, ****P<0.0001 relative to 0 month.
Abbreviations: LC, loading capacity; LNPs, lipid nanoparticles; PDI, polydispersity.
![Figure 3 Characterization of the LNPs suspensions. (A) LNPs size and PDI. Bars represent the size (left y-axis) and dots represent the PDI (right y-axis). (B) LNPs zeta potential. (C) LNPs LC. The parameters were evaluated over time (0 months [dark grey], 3 months [intermediate grey] and 6 months [light grey]).Notes: Values represent the mean±SD of three independently produced formulations. *P<0.05, **P<0.01, ****P<0.0001 relative to 0 month.Abbreviations: LC, loading capacity; LNPs, lipid nanoparticles; PDI, polydispersity.](/cms/asset/b7104113-188b-458e-8400-aeb677723052/dijn_a_12190765_f0003_b.jpg)
Figure 4 In vitro AMX release profiles from the LNPs in three simulated conditions, namely (i) pH 1.6, (ii) pH 5.0, and (iii) pH 7.4.
Notes: Vertical lines represent media changes. Values represent the mean±SD of three independently produced formulations.
Abbreviations: AMX, amoxicillin; LNPs, lipid nanoparticles.
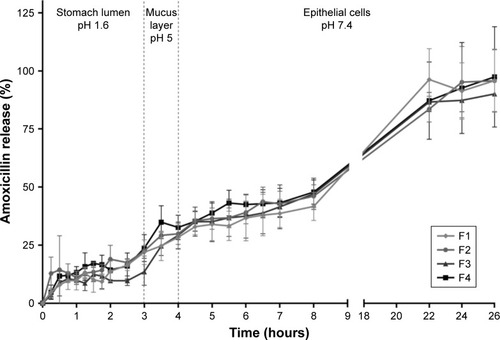
Figure 5 In vitro cell viability studies. (A) L929 cell viability study. (B) MKN-74 cell viability study. Different formulations in different solid lipid concentrations, from 0 (black) to 8 (light gray) mg/mL of solid lipid were evaluated. For free AMX, the same amount of AMX existent in those concentrations of LNPs was used, with the exception of 1 and 4 mg/mL.
Notes: Values represent mean±SD of three independently produced formulations. *P<0.05, **P<0.01, ***P<0.005, ****P<0.0001 relative to 0 mg/mL.
Abbreviations: AMX, amoxicillin; LNPs, lipid nanoparticles.
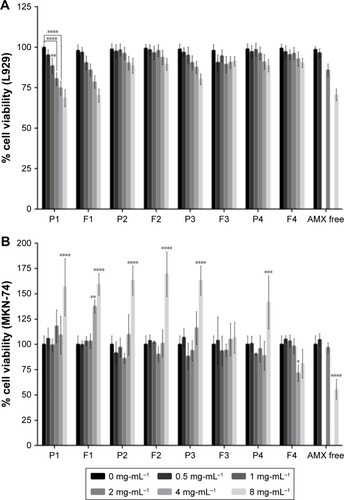
Table 2 Percentage of permeability and apparent permeability (Papp) of the different AMX-loaded LNPs after 3 hours of incubation in different setups: without cells/with mucins; with cells/without mucins; and with cells/with mucins
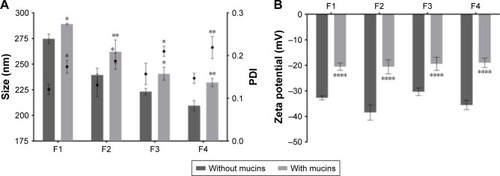
Figure 6 Characterization of the AMX-loaded LNPs suspensions before (dark gray) and after (light gray) the incubation with mucins. (A) LNPs size and PDI. Bars represent the size (left y-axis) and dots represent the PDI (right y-axis). (B) LNPs zeta potential.
Notes: Values represent the mean±SD of three independently produced formulations. *P<0.05, **P<0.01, ****P<0.0001 relative to the LNPs without mucins.
Abbreviations: AMX, amoxicillin; LNPs, lipid nanoparticles; PDI, polydispersity.