Figures & data
Figure 1 (A) Chemical structure of PLGA and (B) celecoxib. Schematic illustrations of formation of PLGA nanoparticles incorporating celecoxib by nanoprecipitation. Celecoxib is molecularly dispersed in the matrix of the nanoparticles.
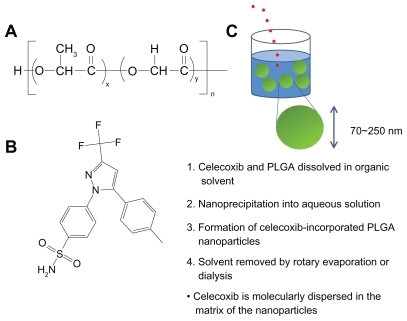
Figure 2 (A) Typical particle size measured by dynamic light scattering and (B) morphology of PLGA nanoparticles incorporating celecoxib (acetone, 40/5 in ) observed by transmission electron microscopy.
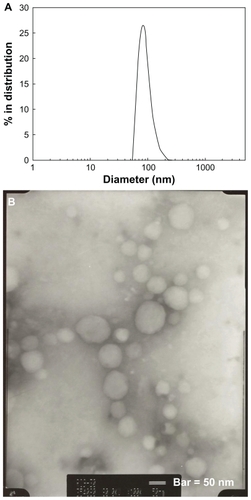
Table 1 Characterization of PLGA nanoparticles incorporating celecoxib according to organic solvent used
Figure 3 X-ray powder diffraction patterns for PLGA nanoparticles incorporating celecoxib. (A) Celecoxib, (B) empty nanoparticles, (C) PLGA nanoparticles incorporating celecoxib (drug content 7.8%, w/w), (D) drug content 10.5%, w/w, and (E) a physical mixture of celecoxib and empty nanoparticles (1/10, w/w).
Note: The data show that celecoxib was molecularly dispersed in the nanoparticle matrix at a lower drug content, and aggregated or crystallized at a higher drug content.
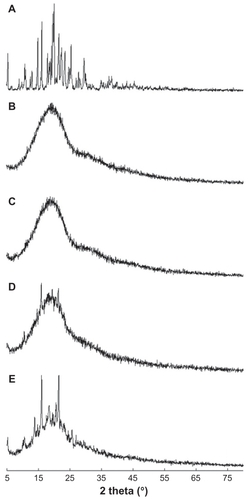
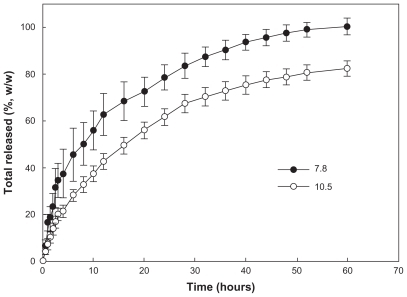
Figure 4 Effect of solvent used on drug release from the PLGA nanoparticles. Solvents used for preparation of the nanoparticles were (A) acetone and tetrahydrofuran and (B) dimethylsulfoxide, dimethylacetamide, 1,4-dioxane, and dimethylformamide. The properties of the PLGA nanoparticles are shown in .
Abbreviations: THF, tetrahydrofuran; DMSO, dimethylsulfoxide; DMAc, dimethylacetamide; DMF, dimethylformamide.
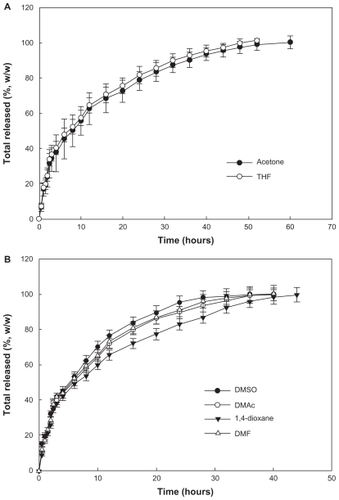
Figure 6 Cytotoxicity of PLGA nanoparticles incorporating celecoxib against brain tumor cells. Different concentrations of celecoxib and PLGA nanoparticles incorporating celecoxib were treated for (A) 1 day, (B) 2 days, and (C) 4 days against U87 tumor cells.
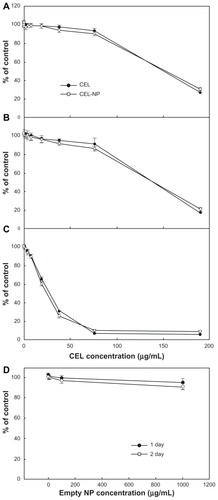
Figure 7 (A) Effect of various concentrations of celecoxib on migration of U87MG cells (in μM). U87MG cells confluently filled a 6 cm culture dish, and half of the area of the cultured cells was removed by a knife following overnight treatment with hydroxyurea and exposed to different concentrations of celecoxib. (B) Comparison of migration ability in an in vitro assay after treatment with a range of celecoxib concentrations.
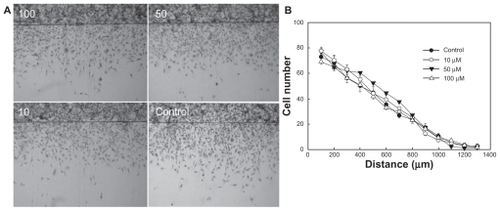
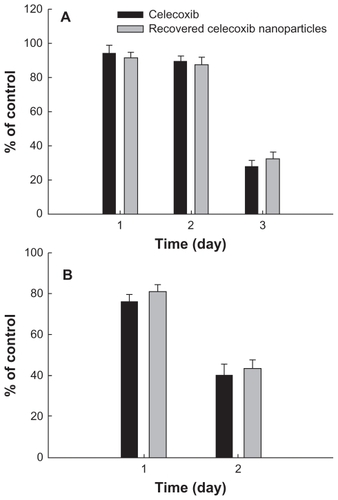
Figure 8 Cytotoxicity of PLGA nanoparticles incorporating celecoxib against C6 rat glioma cells. Different concentrations of celecoxib and PLGA nanoparticles incorporating celecoxib were used for (A) 1 day and (B) 2 days to treat C6 tumor cells.
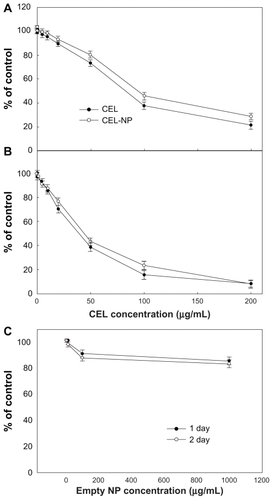
Figure 9 Western blot analysis for COX-2 protein in C6 rat glioma cells. Each lane is as follows: C6 cells treated with celecoxib or PLGA nanoparticles incorporating celecoxib.
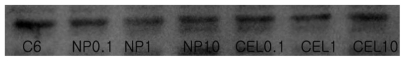
Figure 10 Cytotoxicity of PLGA nanoparticles incorporating celecoxib recovered from drug release experiment against U87MG cells (A) and C6 rat glioma cells (B). During the drug release experiment, PLGA nanoparticles incorporating celecoxib were harvested at 24 hours and dissolved in dimethylsulfoxide, and the harvested celecoxib solution was diluted with cell culture medium. A calculated amount (50 μg/mL) of celecoxib was used to treat the tumor cells.