Figures & data
Figure 1 Chemical structures of CZ48 and its analogs: (A) CZ48; (B) CPT; (C) CZ44.
Abbreviation: CPT, camptothecin.
Figure 2 Response surface plot showing: (A) the influence of the concentration of CZ48 and F-108 on the particle size (nm) of CZ48 nanosuspension formulations; (B) the influence of the concentration of CZ48 and F-108 on the zeta potential (mV) of CZ48 nanosuspension formulations.
Abbreviations: wt%, % of weight; F-108, Pluronic® F108.
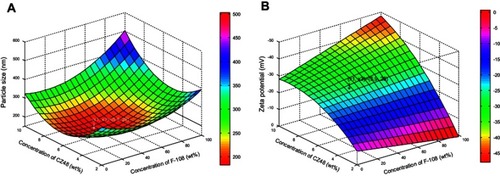
Figure 3 Release profiles of optimal CZ48 nanosuspension formulations in different release media: (A) in PBS (pH 7.4) (n=6); (B) in human plasma (n=6).
Abbreviations: PBS, phosphate buffered saline; NS-L, the nanosuspension with particle size of 589.35 ± 23.27 nm; NS-S, the nanosuspension with particle size of 197.22 ± 7.12 nm.
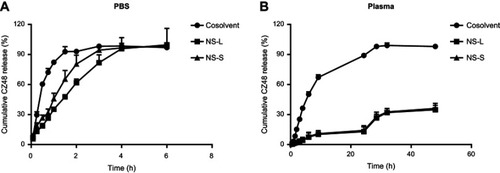
Figure 4 Mean plasma concentration–time profiles of CZ48 (A) and its active metabolite-CPT (B) after i.v. administration of CZ48 cosolvent, NS-S, and NS-L (n=6).
Abbreviations: CPT, campotothecin; i.v., intravenous; NS-L, CZ48 nanosuspension with particle size of 589.35 ± 23.27 nm; NS-S, CZ48 nanosuspension with particle size of 197.22 ± 7.12 nm.
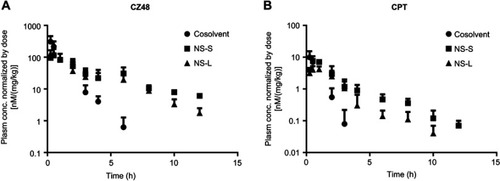
Figure 5 Organ distribution profiles of CZ48 and CPT from cosolvent (A and B), NS-L (C and D) and NS-S (E and F) in mice (n=6).
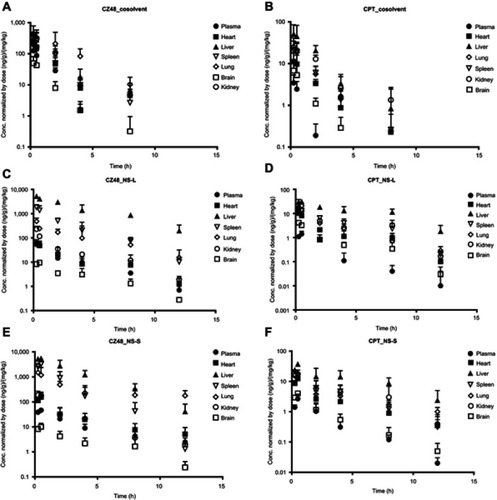
Figure 6 Profiles of partition coefficient (Kp, AUCorgan/AUCplasma) of CZ48 and CPT from cosolvent (A and B), NS-L (C and D) and NS-S (E and F) in mice (n=6).
Figure 7 Average body weight of each group versus the day after the first dose. No statistical difference was observed in the body weights among different groups (n=7 in NT, CP, NP groups, n=10 in Co [5 mg/kg], NS-S-L [5 mg/kg], NS-S-M [25 mg/kg] and NS-S-H [50 mg/kg] groups).
Abbreviations: NT, no treatment; CP, cosolvent placebo; NP, nanosuspension placebo; NS-S-L, NS-S of low dose; NS-S-M, NS-S of medium dose; NS-S-H, NS-S of high dose; NS-S, CZ48 nanosuspension with particle size of 197.22 ± 7.12 nm.
![Figure 7 Average body weight of each group versus the day after the first dose. No statistical difference was observed in the body weights among different groups (n=7 in NT, CP, NP groups, n=10 in Co [5 mg/kg], NS-S-L [5 mg/kg], NS-S-M [25 mg/kg] and NS-S-H [50 mg/kg] groups).Abbreviations: NT, no treatment; CP, cosolvent placebo; NP, nanosuspension placebo; NS-S-L, NS-S of low dose; NS-S-M, NS-S of medium dose; NS-S-H, NS-S of high dose; NS-S, CZ48 nanosuspension with particle size of 197.22 ± 7.12 nm.](/cms/asset/48c399b3-5e23-4e5f-8d2b-cfc1d70e5cff/dijn_a_196453_f0007_b.jpg)
Figure 8 Tumor growth versus time from the first day of dosing to day 29 of treatment period (n=7 in NT, CP, NP groups, n=10 in Co [5 mg/kg], NS-S-L [5 mg/kg], NS-S-M [25 mg/kg] and NS-S-H [50 mg/kg] groups).Abbreviations: NT, no treatment; CP, cosolvent placebo; NP, nanosuspension placebo; NS-S-L, NS-S of low dose; NS-S-M, NS-S of medium dose; NS-S-H, NS-S of high dose; NS-S, CZ48 nanosuspension with particle size of 197.22 ± 7.12 nm.
![Figure 8 Tumor growth versus time from the first day of dosing to day 29 of treatment period (n=7 in NT, CP, NP groups, n=10 in Co [5 mg/kg], NS-S-L [5 mg/kg], NS-S-M [25 mg/kg] and NS-S-H [50 mg/kg] groups).Abbreviations: NT, no treatment; CP, cosolvent placebo; NP, nanosuspension placebo; NS-S-L, NS-S of low dose; NS-S-M, NS-S of medium dose; NS-S-H, NS-S of high dose; NS-S, CZ48 nanosuspension with particle size of 197.22 ± 7.12 nm.](/cms/asset/4010546d-afc4-4f4b-adbc-1fadb9ef291d/dijn_a_196453_f0008_b.jpg)
Figure 9 Percent survival in each group over time in days. The survival was expressed as % surviving from original number at time 0 (n=7 in NT, CP, NP groups, n=10 in Co [5 mg/kg], NS-S-L [5 mg/kg], NS-S-M [25 mg/kg] and NS-S-H [50 mg/kg] groups).
Abbreviations: NT, no treatment; CP, cosolvent placebo; NP, nanosuspension placebo; NS-S-L, NS-S of low dose; NS-S-M, NS-S of medium dose; NS-S-H, NS-S of high dose; NS-S, CZ48 nanosuspension with particle size of 197.22 ± 7.12 nm.
![Figure 9 Percent survival in each group over time in days. The survival was expressed as % surviving from original number at time 0 (n=7 in NT, CP, NP groups, n=10 in Co [5 mg/kg], NS-S-L [5 mg/kg], NS-S-M [25 mg/kg] and NS-S-H [50 mg/kg] groups).Abbreviations: NT, no treatment; CP, cosolvent placebo; NP, nanosuspension placebo; NS-S-L, NS-S of low dose; NS-S-M, NS-S of medium dose; NS-S-H, NS-S of high dose; NS-S, CZ48 nanosuspension with particle size of 197.22 ± 7.12 nm.](/cms/asset/beed5f65-3e20-4d9a-b987-ddf4069be263/dijn_a_196453_f0009_b.jpg)
Table 1 Levels of critical influencing factors and coded correspondent values
Table 2 Experimental responses and the results of central composite design (CCD
Table 3 Predicted values and experimental results of CZ48 nanosuspension prepared under the optimal conditions
Table 4 Pharmacokinetic parameters of CZ48 and active metabolite CPT from cosolvent, NS-S and NS-L after i.v. administration in mice (n=6)
Table 5 CZ48 and CPT organ distribution parameters from cosolvent, NS-S and NS-L in mice after i.v. administration (n=6)
Table 6 Tumor growth rate from the first day of dosing until day 11
Table 7 Summary of significance testing by Kaplan–Meier survival analysis among different groups
Table S1 Effects of different stabilizers and combination with Tween-80 on particle size, PI and zeta potential of nanosuspensions by media milling preparation method
Figure S1 Dependence of particle size (nm) on the milling time (hrs) (n=3), particle size decreased as milling time increase up to 24 hrs.
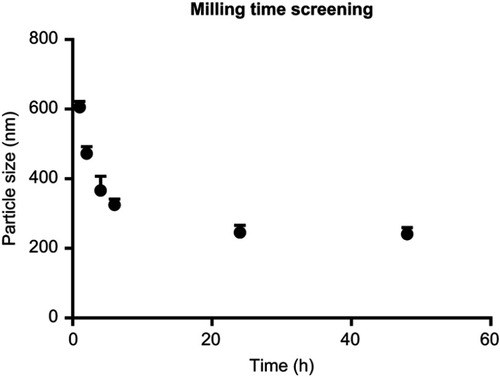
Table S2 Summary of central composite design (CCD) fitting parameters
