Figures & data
Figure 1 Particle size frequencies and solubility of indomethacin in IMC-NPs.
Notes: Particle size frequencies of indomethacin in IMC-MPs (A) and IMC-NPs (B) as determined by a laser diffraction particle size analyzer. (C) Particle size frequencies of indomethacin in IMC-NPs by the dynamic light scattering method. (D) AFM image of indomethacin in IMC-NPs by the SPM-9700. (E) Solubility of indomethacin in IMC-NPs. n=8. *P<0.05 vs IMC-MPs. The ophthalmic formulation containing 35–200 nm sized indomethacin nanoparticles was prepared by treatment with a bead mill. The drug solubility in IMC-MPs and IMC-NPs was higher than that in IMC-MPs at 84.5 µM and 363.2 µM, respectively.
Abbreviations: IMC-MPs, ophthalmic formulation containing indomethacin microparticles; IMC-NPs, ophthalmic formulation containing indomethacin nanoparticles.
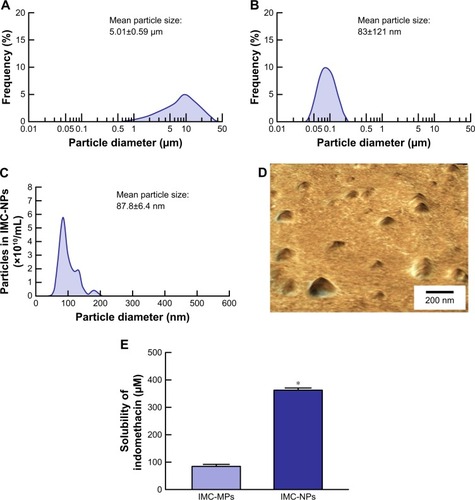
Figure 2 Stability of IMC-NPs 14 days after bead mill treatment.
Notes: Particle size frequencies (A) and AFM image (B) of indomethacin in IMC-NPs by the dynamic light scattering method and SPM-9700, respectively. Changes in concentration (C), particle number (D) and dispersibility (E) of indomethacin in IMC-NPs. n=8. No aggregation or degradation of indomethacin was observed in IMC-NPs, and the size of the indomethacin particles in IMC-NPs remained in the nano order for 14 days.
Abbreviations: IMC-NPs, ophthalmic formulation containing indomethacin nanoparticles; con, concentration.
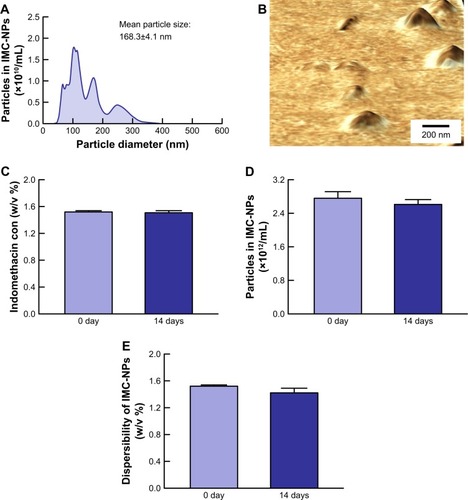
Figure 3 Transepithelial penetration of indomethacin in IMC-NPs at 4°C and 37°C using HCE-T cell monolayers.
Notes: (A) Changes of TER in HCE-T cell monolayers treated with IMC-NPs 4°C and 37°C. Accumulation (B) and penetration (C) of indomethacin in HCE-T cells treated with IMC-solution, IMC-MPs and IMC-NPs, at 4°C and 37°C. Number (D) and size frequencies (E) of indomethacin nanoparticles in the basolateral side at 4°C conditions. n=6. *P<0.05 vs 37°C conditions for each category. #P<0.05 vs IMC solution at 37°C conditions. The accumulation of indomethacin tended to be less at 4°C, and transepithelial penetration was significantly prevented at 4°C.
Abbreviations: IMC-MPs, ophthalmic formulation containing indomethacin microparticles; IMC-NPs, ophthalmic formulation containing indomethacin nanoparticles; con, concentration; TER, transepithelial electrical resistance.
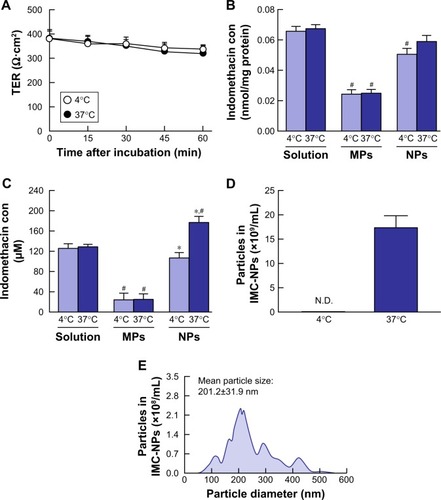
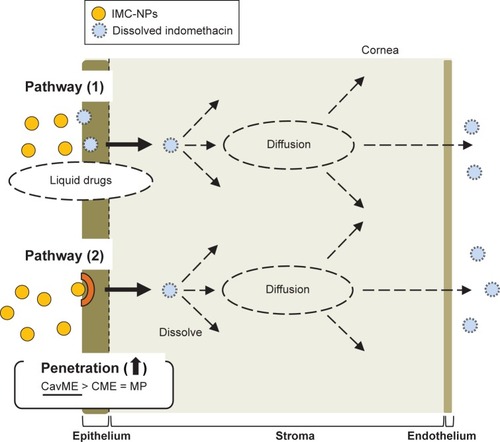
Figure 4 Effect of endocytosis pathways on transepithelial penetration of IMC-NPs in HCE-T cell monolayers.
Notes: Effects of endocytosis inhibitors on the TER (A), accumulation (C) and penetration (E) of indomethacin in HCE-T cell monolayers treated with IMC-NPs. Changes in TER (B), accumulation (D) and penetration (F) of indomethacin in HCE-T cell monolayers treated with IMC-NPs by multi-treatment with nystatin, dynasore and rottlerin (Nys+Dyn+Rot). (G and H) Effect of endocytosis inhibitors on the number of indomethacin nanoparticles in the basolateral side. n=5–8. *P<0.05 vs vehicle for each category. The penetration of indomethacin was significantly decreased by the combination of nystatin, dynasore and rottlerin.
Abbreviations: IMC-NPs, ophthalmic formulation containing indomethacin nanoparticles; con, concentration.
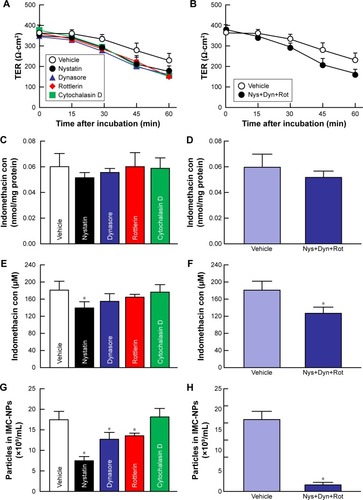
Table 1 Pharmacokinetic analysis of the penetration of IMC-NPs in rabbit corneas treated at cold temperature (4°C) or with endocytosis inhibitors
Figure 5 Effect of endocytosis pathways on in vitro transcorneal penetration of IMC-NPs in rabbit corneas.
Notes: (A) Penetration profile of indomethacin in IMC-NPs at 4°C and 37°C. (B) AUCpenetration of indomethacin in IMC-NPs at 4°C and 37°C. Penetration profile (C) and AUCpenetration (D) of indomethacin in IMC-NPs by treatment with individual endocytosis inhibitors. Penetration profile (E) and AUCpenetration (F) of indomethacin in IMC-NPs by multi-treatment with nystatin, dynasore and rottlerin (Nys+Dyn+Rot). n=5–8. *P<0.05 vs 37°C. #P<0.05 vs vehicle for each category. AUCpenetration was significantly decreased by the combination of nystatin, dynasore and rottlerin, and the decreased AUCpenetration levels were similar to those at 4°C in rabbit cornea.
Abbreviations: AUCpenetration, area under the drug concentration–time curve in the reservoir chamber; con, concentration; IMC-NPs, ophthalmic formulation containing indomethacin nanoparticles.
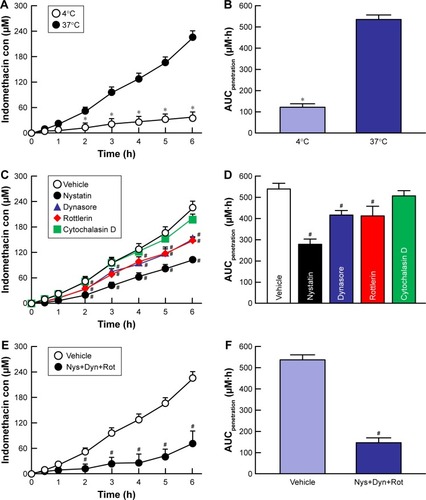
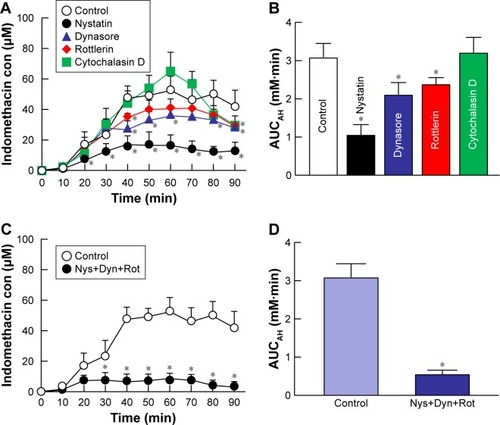
Figure 6 Effect of endocytosis pathways on in vivo transcorneal penetration of IMC-NPs in rabbit corneas.
Notes: Intraocular behavior (A) and AUCAH (B) of indomethacin in IMC-NPs by treatment with individual endocytosis inhibitors. Intraocular behavior (C) and AUCAH (D) of indomethacin in IMC-NPs by multi-treatment with nystatin, dynasore and rottlerin (Nys+Dyn+Rot). n=4–6. *P<0.05 vs control for each category. Dynasore and rottlerin tended to decrease the AUCAH. On the other hand, nystatin significantly prevented transcorneal penetration. In addition, the AUCAH in rabbit cornea with multi-treatment was obviously lower than that in rabbit cornea treated with each individual endocytosis inhibitor.
Abbreviations: AUCAH, area under the drug concentration–time curve in the aqueous humor; con, concentration; IMC-NPs, ophthalmic formulation containing indomethacin nanoparticles.