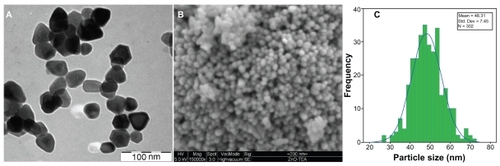
Figures & data
Figure 1 The schematic image of formation of the zinc oxide nanoparticles from the zinc oxide seed, and the role of triethanolamine as a polymerization agent.
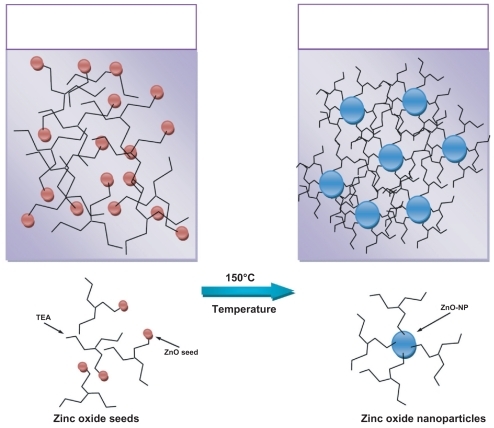
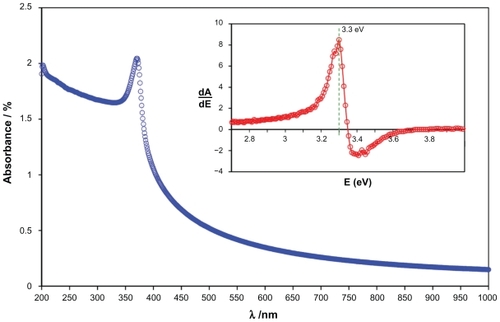
Figure 2 The Fourier transform infrared spectroscopy pattern of the zinc oxide nanoparticles prepared by the solvothermal method at 150°C.
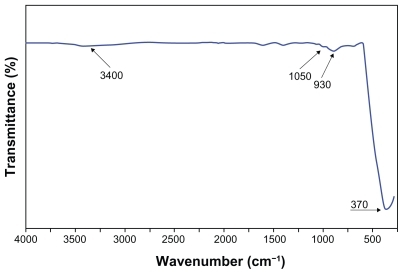
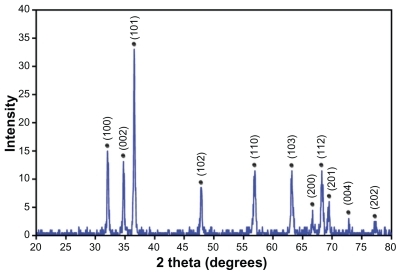
Figure 3 The X-ray diffraction pattern of the zinc oxide nanoparticles prepared by the solvothermal method at 150°C.