Figures & data
Figure 1 Characterization of Se nanoparticles. TEM imaging (A) shows a near-spherical shape of the nanoparticles with major sizes about 30–70 nm. High-resolution TEM (B) shows the stabilizing PVA layer (b) adsorbed on the core Se (a) which appears to be crystalline [c] showing the direction of crystal planes). (C) XPS analysis showing Se 3d peaks at 55.4 and 56.2, suggesting its zero oxidation state.
![Figure 1 Characterization of Se nanoparticles. TEM imaging (A) shows a near-spherical shape of the nanoparticles with major sizes about 30–70 nm. High-resolution TEM (B) shows the stabilizing PVA layer (b) adsorbed on the core Se (a) which appears to be crystalline [c] showing the direction of crystal planes). (C) XPS analysis showing Se 3d peaks at 55.4 and 56.2, suggesting its zero oxidation state.](/cms/asset/63f8099a-0fb0-4d1d-a3f3-b6624f9d2a7e/dijn_a_12190809_f0001_b.jpg)
Figure 2 In vitro testing results against MRSA. (A) OD growth curves of bacteria solutions treated with increasing amount of Se from 0.25 ppm to 128 ppm showing reduced growth of bacteria treated with the Se NPs. (B) Live/dead assay results showing reduced live cell numbers when bacteria were treated with increasing concentrations of Se NPs. (C1, D1, and E1) Representative flow cytometry 2D dot plots of bacteria, treated with 0 ppm Se (ie, untreated), 0.5 ppm Se and 128 ppm Se and corresponding histograms (C2, D2, and E2).
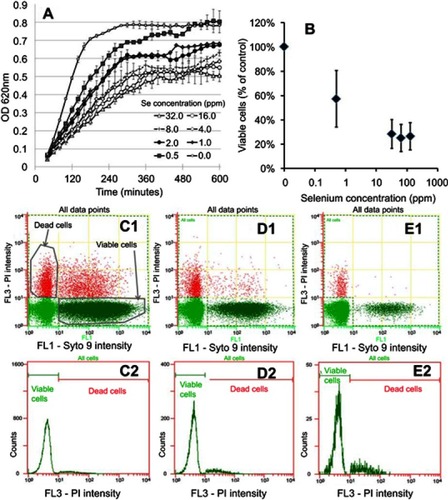
Figure 3 In vitro testing results against MRSE. (A) OD growth curves of bacteria solutions treated with increasing amounts of Se NPs from 0.25 ppm to 256 ppm showing reduced growth when bacteria were treated with Se. (B) Live/dead assay results showing reduced live cell numbers when bacteria were treated with increasing concentrations of Se NPs. (C1, D1, and E1) Flow cytometry 2D dot plots of bacteria, treated with 0 ppm Se (ie, untreated), 0.5 ppm Se and 128 ppm Se and corresponding histograms (C2), (D2), and (E2).
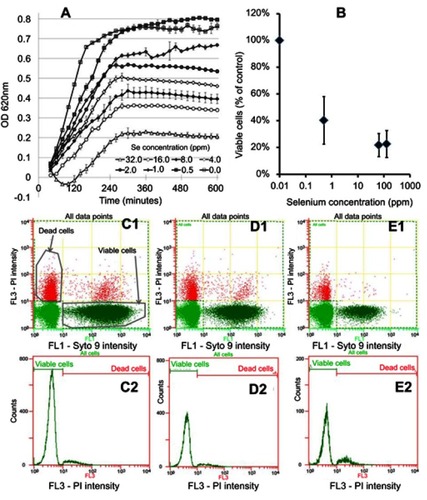
Figure 4 Representative images of the titanium plates and screws used in in vivo experiments. (A and B) are low-magnification SEM images of a coated plate and screw, respectively. (C) is a photograph of an uncoated plate and screw. Coated plates and screws showed the same gross appearance and microscopically smooth surface (A and B). (E and F) are SEM images of coated plate and coated screw surfaces, respectively, showing the clear presence of Se nanoparticles compared to an uncoated surface (D).
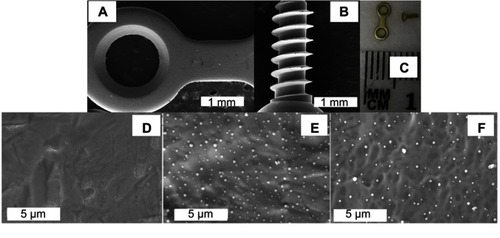
Figure 5 In vitro biocompatibility results of osteoblasts cultured on Ti and Se-coated Ti substrates. (A) Schematic of titanium (Ti) and titanium-selenium (Ti-Se) plates being seeded with human primary osteoprogenitor cells. (B) Metabolic activity over 7 days is similar between Ti and Ti-Se plates. (C) Morphology of primary osteoprogenitor cells after 1 and 7 days post seeding shows the typical elongated phenotypes and full coverage of the plates, without differences between substrates.
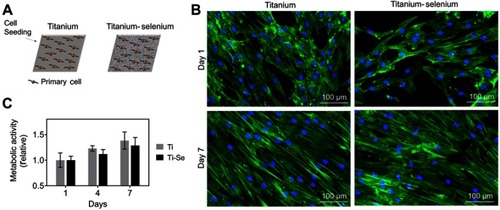
Figure 6 Numbers of colony forming units (CFU) retrieved from wound pocket swabs and screws 4 weeks after implantation showing decreased CFU counts with the Se NP coating. Data = mean ± SEM (n=3); * p<0.05.
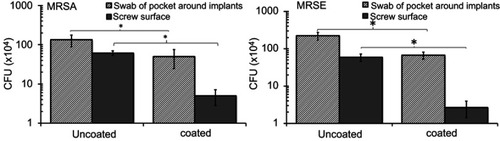
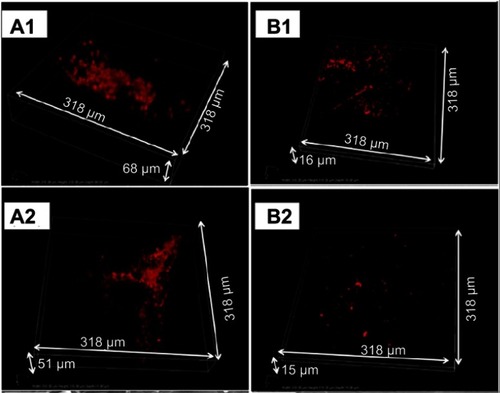
Figure 7 In vivo biofilm formation by MRSA (A1 and B1) and MRSE (A2 and B2) on implanted plates. Representative confocal microscopy snapshots of 3D reconstructions (obtained with a 40X objective) of immunostained bacteria on uncoated (A1 and A2) and coated (B1 and B2) plates after 4 weeks of implantation.