Figures & data
Table 1 Immunosuppressive agents commonly used in transplantation
Figure 1 Factors influencing the bioavailability of oral tablet sirolimus. Generally, the bioavailability of oral tablet sirolimus is determined by the solubility and permeability of sirolimus. The nanocrystalline sirolimus improves sirolimus dissolution, saturation solubility, and stability in gastrointestinal (GI) lumen and thereby improves the aborption. However, sirolimus is the substrate for the metabolic enzyme (cytochrome P450 3A) and efflux transporter (P-glycoprtoein) in intestinal and hepatic cells. Therefore, less than 20% of sirolimus can reach systemic circulation.
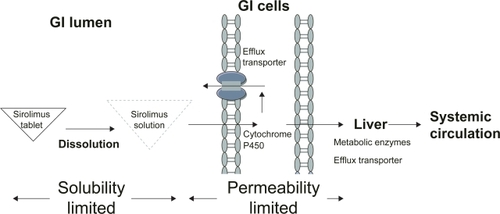
Figure 2 The nanocrystalline sirolimus system. Generally recognized as safe (GRAS) stabilizers were milled with sirolimus into nanocrystal particles by NanoCrystal® technology.
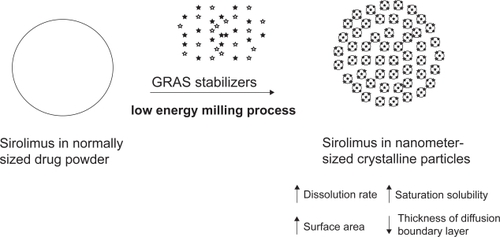
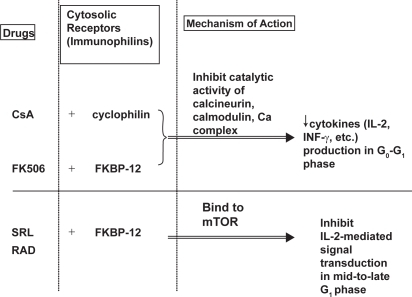
Figure 3 Mechanism of action of cyclosporine, tacrolimus, and sirolimus. Adapted with permission from CitationWu FL, Tsai MK, et al. 2005. Effects of conversion from sirolimus oral solution to tablets in stable Taiwanese renal transplant recipients. J Formos Med Assoc, 104:22–8. © The Formosan Medical Association.
Abbreviations: CsA, cyclosporine; Ca, calcium; FK506, tacrolimus; KBP, FK binding protein; INF-γ, γ-interferon; IL, interleukin; mTOR; mammalian target of rapamycin; RAD; everolimus; SRL, sirolimus.