Figures & data
Figure 1 Fullerol could reduce TNF-α, IL-1β, IL-6 and iNOS expression and nitrite production in primary mouse peritoneal macrophages.
Notes: Fullerol was not toxic at doses of up to 3 µM (A, WST-1 assay). Fullerol suppressed LPS-induced nitrite production (B, Griess reagent test) and iNOS gene expression (C, quantitative RT-PCR analysis). It also reversed LPS-stimulated production of TNF-α (D, ELISA assay; E, quantitative RT-PCR analysis) and expression of inflammatory cytokines IL-1β and IL-6 (F, quantitative RT-PCR analysis). LPS: LPS (100 ng/mL) treatment; LPS + FUL: treatment of LPS (100 ng/mL) combined with fullerol (1 µM).
Abbreviations: FUL, fullerol; NT, no treatment; iNOS, induced nitric oxide synthase; LPS, lipopolysaccharide; TNF, tumor necrosis factor.
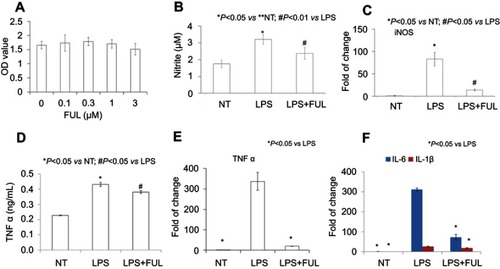
Figure 2 Fullerol could decrease nitrite production and TNF-α, IL-6 and IL-1β expression in RAW264.7 macrophage cell line.
Notes: Fullerol was not toxic at the doses of up to 3 µM (A, WST-1 assay). Fullerol suppressed LPS-induced nitrite production (B, Griess reagent test) and iNOS gene expression (C, quantitative RT-PCR analysis). It also reversed LPS-stimulated production of TNF-α (D, ELISA assay; E, quantitative RT-PCR analysis) and expression of inflammatory cytokines IL-1β and IL-6 (F, quantitative RT-PCR analysis). LPS: LPS (100 ng/mL) treatment; LPS + FUL: treatment of LPS (100 ng/mL) combined with fullerol (1 µM); LPS + DEX: treatment of LPS (100 ng/mL) combined with dexamethasone (25 µM). For all assays, four repeats were performed in each group (n=4).
Abbreviations: FUL, fullerol; NT, no treatment; iNOS, induced nitric oxide synthase; LPS, lipopolysaccharide; TNF, tumor necrosis factor.
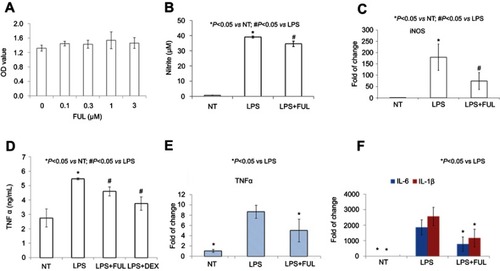
Figure 3 Fullerol suppresses cell fusion and formation of multinuclear cells in RAW264.7 macrophage cell line.
Notes: Cells were stained with the FITC-conjugated phalloidin, a peptide with high ability of binding to the microfilament component F-actin. (A) Images were taken under a fluorescent microscope; (B) numbers of the multinuclear cells in a randomly selected area consisting of 50 nuclei in cell cultures with different treatments. Cell fusion which led to the formation of multinuclear was induced by LPS, and the induction effect of LPS was inhibited with the addition of fullerol. LPS: LPS (100 ng/mL) treatment; LPS + FUL: treatment of LPS (100 ng/mL) combined with fullerol (1 µM).
Abbreviations: FUL, fullerol; NT, no treatment; LPS, lipopolysaccharide.
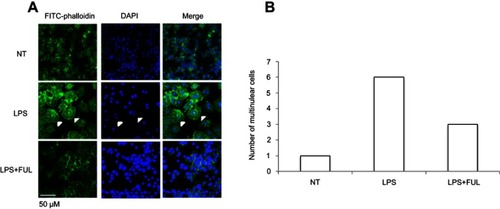
Figure 4 Fullerol decreases protein levels of TLR4 (A) and p-P38 (C) in the cytosol fraction and NFkB (B) and FoxO1 (D) in the nuclear fraction determined by western blot. Cells were treated by 100 ng/mL LPS alone (LPS) or 100 ng/mL LPS together with 1 µM fullerol (LPS + FUL). It was shown that protein levels of these signal molecules were significantly lower in LPS group than in LPS + FUL group. *P<0.05, n=3.
Abbreviations: FUL, fullerol; FoxO1, forkhead box transcription factor 1; LPS, lipopolysaccharide; NFkB, nuclear factor-kappaB; TLR, toll-like receptor.
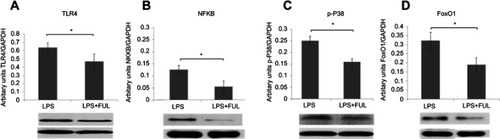
Figure 5 Representative H&E staining of synovium from rat knee joints (control: PBS, injured: MIA) with (FUL) or without (NT) fullerol treatment for 5 days.
Notes: Fullerol of 2 mg/kg weight was administered intravenously immediately after MIA injection. The results showed that fullerol reduces red blood cells (blue arrows) and fibroblast-like cells (black arrows) in the synovium of the injured knee seen in the up/left image of the MIA/NT group.
Abbreviations: FUL, fullerol; MIA, monoiodoacetate; NT, no treatment.
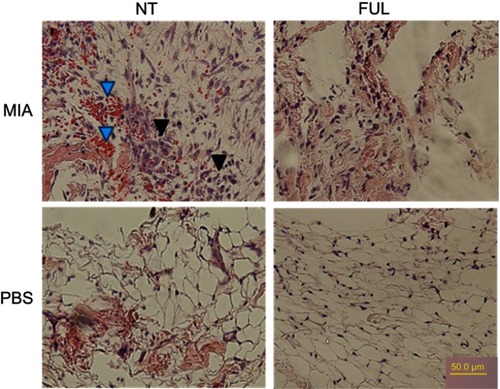
Figure 6 Representative Safranin O/Fast green staining of rat knee joints (A, control: PBS, injured: MIA) and femoral articular cartilages (B) in both joints with (Ful) or without (NT) fullerol treatment.
Notes: Osteoarthritis was induced in the knee joints by intra-articular injection of MIA for up to 21 days. It was shown that MIA-induced histopathological alterations, including a decrease in the matrix production and the thickness of the articular cartilage, erosion of the articular cartilage, disorientation of the chondrocytes, fibrillation of the superficial zone and exposure of the subchondral bone, were time-dependent (A). Fullerol alleviates the loss of matrix, chondrocytes (black arrows) and cartilage thickness (black lines and the bar chart) in the femoral articular cartilage (B). Abbreviations: MIA, monoiodoacetate; NT, no treatment.
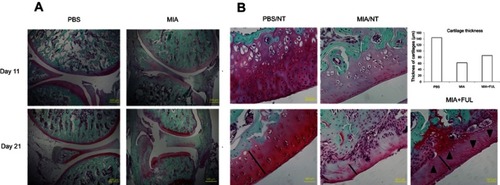
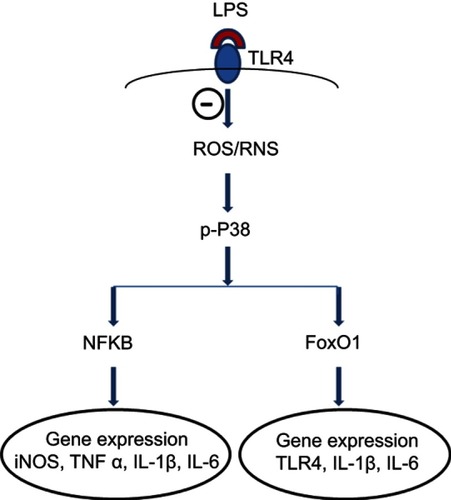
Figure 7 A proposed mechanism underlying inhibitory effects of fullerol on inflammation-related gene expression in LPS-activated macrophages. The symbol ⊝ represents inhibition of fullerol against free radicals produced after binding of LPS to its ligand TLR4. The p38 MAPK plays a pivotal role in the regulation. Through transcription factors, NFkB and FoxO1, the expression of targeted genes was regulated. The decrease in iNOS and TLR4 expression reduces the cellular level of reactive nitrogen species (RNS) and TLR4 protein, which further minimizes the inflammatory effects of LPS. It is worth noting that other signal pathways might also be involved in the anti-inflammation of fullerol.
Abbreviations: FoxO1, forkhead box transcription factor 1; iNOS, induced nitric oxide synthase; LPS, lipopolysaccharide; NFkB, nuclear factor-kappaB; TLR, toll-like receptor.