Figures & data
Table 1 Mechanisms of ENM-induced neurotoxicity in vivo
Table 2 Mechanisms of ENM-induced neurotoxicity in vitro
Table 3 Assays for DNA damage and corresponding results
Figure 1 Possible ways in which engineered nanomaterials (ENMs) cross the blood–brain barrier (BBB) and the potential risks. ENMs with specific physicochemical properties could pass through the BBB by way of several different strategies.Notes: 1) Transcellular diffusion. ENMs with low molecular weight, eg, solid lipid nanoparticles, can pass through the BBB in this way. 2) Paracellular diffusion. Some ENMs, eg, silica nanoparticles and reduced graphene oxide, can open paracellular spaces, and then get into the central nervous system (CNS). 3) Receptor-mediated transcytosis. ENMs with ligands like transferrin, insulin, ApoE, etc can be identified by corresponding receptors on the endothelial cells. 4) Adsorptive mediated transcytosis. ENMs with positive charges can be attracted by microvascular endothelial cells that are negatively charged. 5) Cell-mediated transcytosis. Macrophages with phagocytized ENMs in the blood could pass through the BBB, and release ENMs into the CNS.
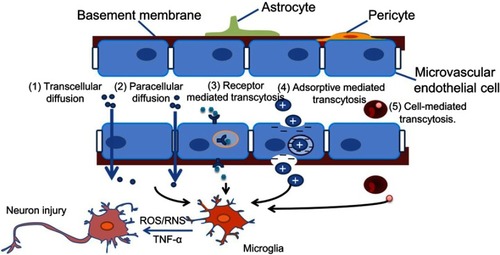
Figure 2 Mechanisms of ROS production induced by engineered nanomaterials (ENMs). ROS have different sources.Notes: 1) Oxidative burst. ENM-activated microglia can produce ROS with the catalyzation of NADPH-oxidase. 2) Mitochondrial ROS. Disruption of electron transport chain will lead to significant increase of ROS. 3) Fenton or Fenton-like reaction. The transition metals, eg, iron, copper, chromium, and cobalt can mediate the formation of ROS like highly reactive hydroxyl radical (OH.) through Fenton reaction. 4) Enzyme-like activities of ENMs. For example, the INOPs could behave like peroxidase. (5) Lysosome membrane permeabilization (LMP). LMP could produce ROS through lysosomal iron or by causing mitochondrial membrane permeabilization and producing mitochondrial ROS.
Abbreviation: IONPs, iron oxide nanoparticles.
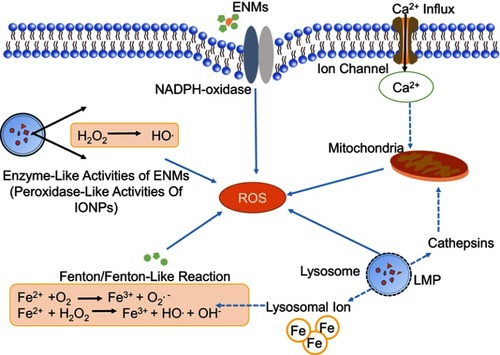
Figure 3 Possible mechanisms of releasing pro-inflammatory cytokines in microglia. Engineered nanomaterials (ENMs) can provoke TLRs like TLR2 and TLR4. TLR4 can further activate NF-κB pathway, which results in the release of inflammatory cytokines. ENMs can also activate ERK1/2 by inhibiting MKPs (MAPK phosphatases), and then cause the release of inflammatory cytokines.
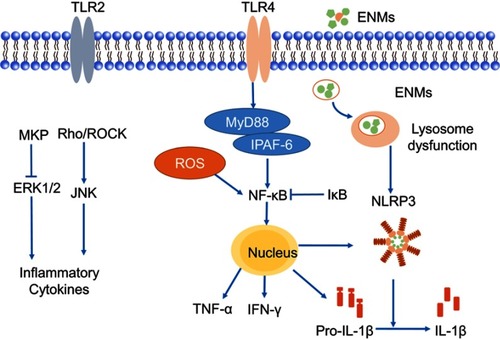
Figure 4 Mechanisms of engineered nanomaterials (ENMs)-induced DNA damage. ENMs could cause different types of DNA damage including DNA crosslinks, single/double strand breaks, and DNA adducts. DNA damage could lead to cell cycle arrest to provide enough time for DNA repair and inefficient DNA repair could induce apoptosis, senescence, and cancer.
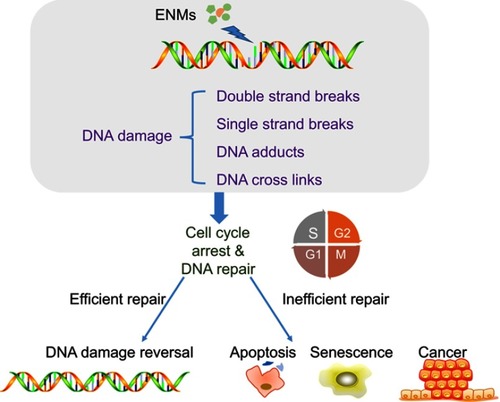
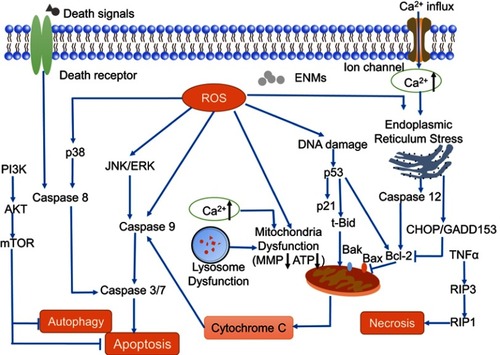
Figure 5 Mechanisms of engineered nanomaterials (ENMs)-induced cell death in neurotoxicity. Apoptosis is a caspase-dependent cell death, which has three main pathways including death receptor pathway, mitochondrial pathway, and endoplasmic reticulum stress pathway. Autophagy could be negatively mediated by PI3K-Akt-mTOR pathway. For necrosis, RIP3 responding to TNF family of cytokines binds to the kinase RIP1 and is crucial in the programmed necrosis pathway.