Figures & data
Table 1 Summary of the simulated systems
Table 2 Composition and physical properties of moeixitecan-loaded liposomal nanoparticles (MLP)
Figure 1 The characterization and physical stability of moeixitecan-loaded liposomal nanoparticle (MLP). (A) Schematic illustration of the SN38 prodrug, MLP. (B) Morphological characteristics of MLP. (C) Particle size distribution of MLP. (D) Size changes of MLP were examined as a function of time at 4°C for 3 months. (E) Size changes of MLP in different medium were examined for 7 days.
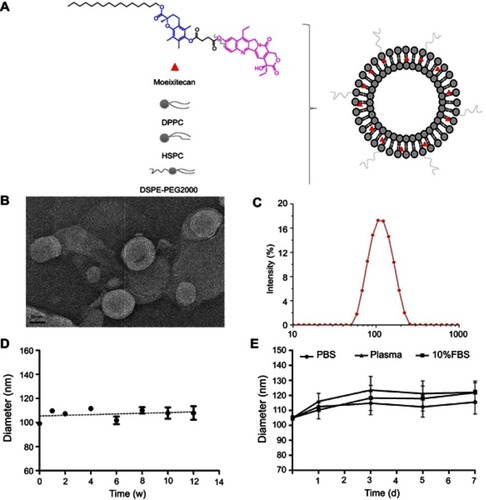
Figure 2 SN38 released from moeixitecan (M) solution and MLP at 37°C in PBS (pH 7.4) containing 0.2% Tween 80 at 37°C.
Abbreviation: MLP, moeixitecan-loaded liposomal nanoparticle.
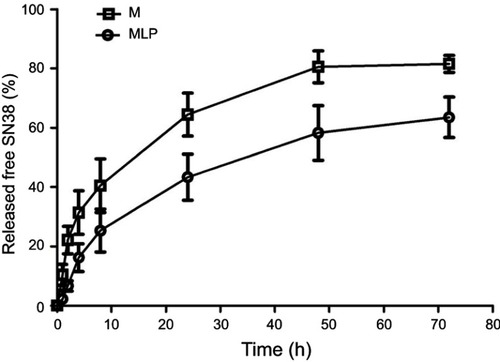
Figure 3 Molecular dynamics simulations of dipalmitoyl phosphatidylcholine (DPPC) bilayers with moeixitecan. (A) Snapshots of the systems consisting of moeixitecan molecules in DPPC bilayers. (B) Schematics illustrating the proposed locations of moeixitecan in the lipid membrane. (C) Mass density profiles of moeixitecan and the selected lipid atoms along the bilayer. (D) The thickness of the membrane with increasing concentrations of moeixitecan. (E) The areas per molecule of lipid bilayer with increasing concentrations of moeixitecan.
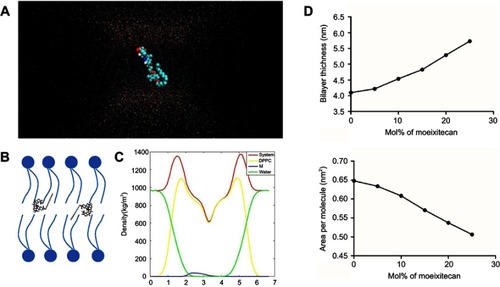
Figure 4 (A and B) The cytotoxicity of CPT-11, moeixitecan (M), and moeixitecan-loaded liposomal nanoparticles (MLP) toward HT-29 cells for 24 and 48 hrs. (C) Flow cytometry analysis for apoptosis of HT-29 cells in induced by control, CPT-11, M, and MLP at the same SN38 equivalent concentration for 24 and 48 hrs.
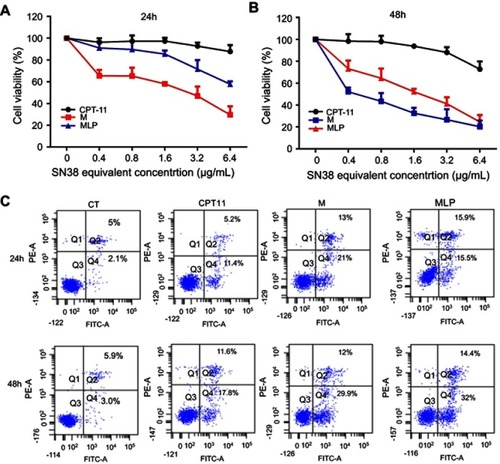
Table 3 The main pharmacokinetic parameters in SD rats after intravenous injection of CPT-11, moeixitecan (M) ,and moeixitecan-loaded liposomal nanoparticles (MLP) (n=3)
Figure 5 In vivo plasma concentration-time profiles of SN38 after intravenous injection of CPT-11, moeixitecan (M), and moeixitecan-loaded liposomal nanoparticles (MLP) in SD rats (n=3).
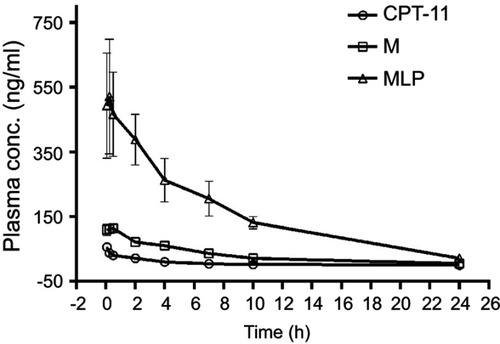
Figure 6 Biodistribution of ir623 and iMLP in HT-29 tumor-bearing mice. (A) Representative images of in vivo whole-body imaging of mice at 0.5, 4, 8, 24, and 48 hrs postintravenous injection of ir623 and iMLP. (B) The ex vivo optical images of the tumors and other major organs from the sacrificed mice (n=3).
Abbreviation: iMLP, iDSPE-embeded moeixitecan-loaded liposomal nanoparticles.
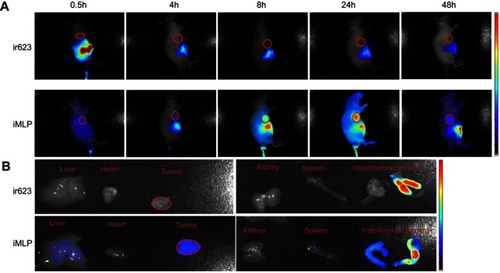
Figure 7 Anti-tumor effect on HT-29 tumor-bearing mice treated with moeixitecan-loaded liposomal nanoparticles (MLP). (A) Tumor volume changes as a function of time after intravenous injection of CPT-11, moeixitecan (M), and MLP. (B) Body weight profile of tumor-bearing mice after treatment. (C) Typical excised tumors at the time of sacrifice. (D) Statistical results for tumor weight.
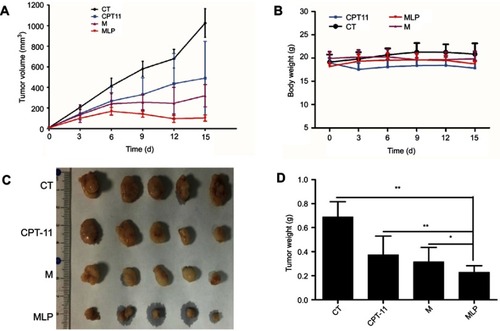
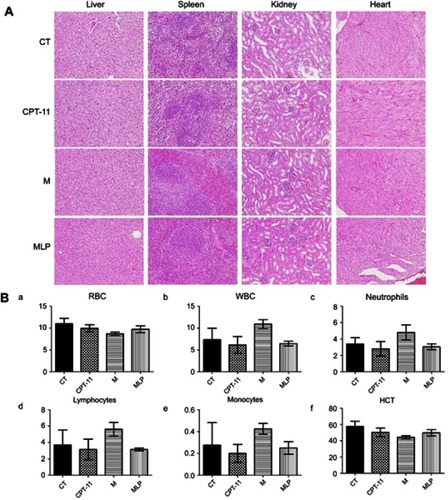
Figure 8 Safety evaluation of moeixitecan-loaded liposomal nanoparticles (MLP).
Notes: (A) H&E stained images of organ sections separated from mice. Magnification, ×200 (B) blood parameters panel data from mice injected i.v. with saline (control, CT), CPT-11, moeixitecan (M), and moeixitecan-loaded liposomal nanoparticles (MLP). Mean parameters value±SD, n=5. RBC count (a), WBC count (b), neutrophil count (c), lymphocyte count (d), monocyte count (e), and HCT value (f).
Abbreviations: WBC, white blood cell; RBC, red blood cell; HCT, hematocrit.