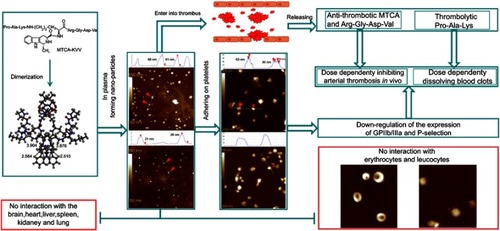
Figures & data
Figure 1 Preparation of MTCA-KKV. (i) DCC, HOBt, NMM and THF; (ii) H2, Pd/C and CH3OH; (iii) hydrogen chloride in ethyl acetate (4 M); (iv) H2SO4 (5 M), 60ºC; (v) DMF, (Boc)2O and trimethylamine.
Abbreviations: DCC, dicyclohexylcarbodiimide; HOBt, N-hydroxybenzotriazole; NMM, N-methylmorpholine; THF, tetrahydrofuran; (Boc)2O, ditertbutyl dicarbonate; DMF, NN-dimethylformide; MTCA, (1R,3S)-1-methyl-1,2,3,4-tetrahydro-β-carboline-3-carboxylic acid; MTCA-KKV, (1R,3S)-1-methyl-1,2,3,4-tetrahydro-β-carboline-3-carboxyl-Lys(Pro-Ala-Lys)-Arg-Gly-Asp-Val.
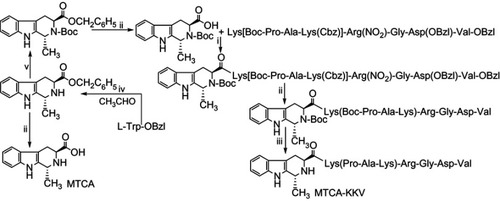
Figure 2 FT-ICR-MS, qCID and NOESY 2D NMR spectra of MTCA-KKV. (A) FT-ICR-MS spectrum of MTCA-KKV in aqueous solution; (B) qCID spectrum of MTCA-KKV in aqueous solution;(C) NOESY 2D NMR spectrum of MTCA-KKV in DMSO-d6; (D) energy minimized conformation of the dimer of MTCA-KKV.
Abbreviations: MTCA-KKV, (1R,3S)-1-methyl-1,2,3,4-tetrahydro-β-carboline-3-carboxyl-Lys(Pro-Ala-Lys)-Arg-Gly-Asp-Val.
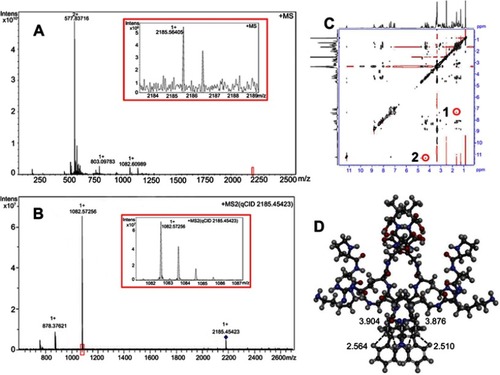
Figure 3 Tyndall effect, particle size and zeta potential of MTCA-KKV in ultrapure water. (A) Feature of ultrapure water of pH 6.7; (B) Feature of 0.1 μM solution of MTCA-KKV in ultrapure water of pH 6.7; (C) Feature of ultrapure water of pH 6.7 with the radiation of 650 nm laser; (D) Feature of 0.1 μM solution of MTCA-KKV in ultrapure water of pH 6.7 with the radiation of 650 nm laser; (E) Feature of ultrapure water of pH 2.0; (F) Feature of 0.1 μM solution of MTCA-KKV in ultrapure water of pH 2.0; (G) Feature of ultrapure water of pH 2.0 with the radiation of 650 nm laser; (H) Feature of 0.1 μM solution of MTCA-KKV in ultrapure water of pH 2.0 with the radiation of 650 nm laser; (I) Seven-days’ size of MTCA-KKV in pH 6.7 and pH 2.0 ultrapure water (0.1 μM); (J) Zeta potential of MTCA-KKV in pH 6.7 ultrapure water (0.1 μM).
Abbreviations: MTCA-KKV, (1R,3S)-1-methyl-1,2,3,4-tetrahydro-β-carboline-3-carboxyl-Lys(Pro-Ala-Lys)-Arg-Gly-Asp-Val.
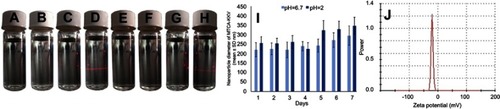
Figure 4 TEM, SEM and AFM images of MTCA-KKV in ultrapure water and plasma of rat. (A and B) TEM image and size distribution of particles of MTCA-KKV in ultrapure water of pH 6.7 (10.0 μM); (C and D) TEM image and size distribution of particles of MTCA-KKV in ultrapure water of pH 6.7 (0.1 μM); (E and F) TEM image and size distribution of particles of MTCA-KKV in ultrapure water of pH 6.7 (1.0 nM); (G and H) SEM image and size distribution of powders lyophilized from a solution of MTCA-KKV in pH 6.7 ultrapure water (10.0 μM); (I and J) SEM image and size distribution of powders lyophilized from a solution of MTCA-KKV in pH 6.7 ultrapure water (0.1 μM); (K and L) SEM image and size distribution of powders lyophilized from a solution of MTCA-KKV in pH 6.7 ultrapure water (1.0 nM); (M) AFM image of rat plasma; (N, O and P) AFM image and size distribution of the particles of MTCA-KKV in rat plasma (10.0 μM, 0.1 μM and 1.0 nM); (Q) MTCA-KKV’s nanoparticle predicted with mesoscale simulation software.
Abbreviations: TEM, transmission electron microscopy; SEM, scanning electron microscopy; AFM, atomic force microscopy; MTCA-KKV, (1R,3S)-1-methyl-1,2,3,4-tetrahydro-β-carboline-3-carboxyl-Lys(Pro-Ala-Lys)-Arg-Gly-Asp-Val.
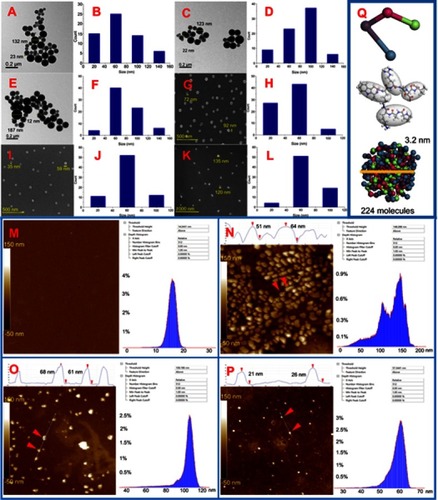
Figure 5 UV and CD described in vitro interaction between MTCA-KKV and P-selectin or GPIIb/IIIa. (A) P-selectin concentration dependently changes the UV of MTCA-KKV; (B) GPIIb/IIIa concentration dependently changes the UV MTCA-KKV; (C) MTCA-KKV concentration dependently changes the CD of P-selectin; (D) MTCA-KKV concentration dependently changes the CD of GPIIb/IIIa.
Abbreviations: UV, ultra violet spectrum; CD, circular dichroism spectrum; MTCA-KKV, (1R,3S)-1-methyl-1,2,3,4-tetrahydro-β-carboline-3-carboxyl-Lys(Pro-Ala-Lys)-Arg-Gly- Asp-Val.
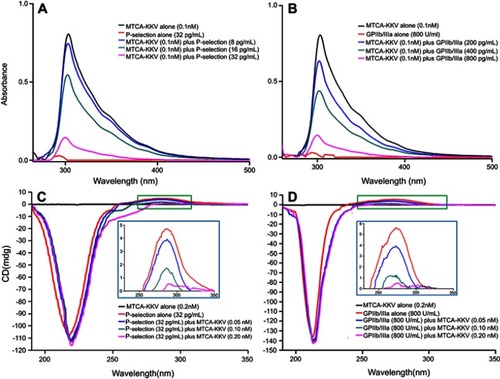
Figure 6 AFM feature of rat platelets, erythrocytes and leucocytes with and without MTCA-KKV. (A) AFM feature of rat platelets alone; (B) AFM feature of rat platelets with MTCA-KKV (0.1 nM, in pH 6.7 ultrapure water); (C) AFM feature of rat erythrocytes alone; (D) AFM feature of rat erythrocytes with MTCA-KKV (0.1 nM, in pH 6.7 ultrapure water); (E) AFM feature of rat leucocytes alone; (F) AFM feature of rat leucocytes with MTCA-KKV (0.1 nM, in pH 6.7 ultrapure water).
Abbreviations: AFM, atomic force microscopy; MTCA-KKV, (1R,3S)-1-methyl-1,2,3,4-tetra-hydro-β-carboline-3-carboxyl-Lys(Pro-Ala-Lys)-Arg-Gly-Asp-Val.
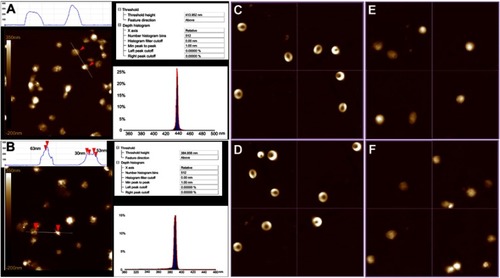
Figure 7 In vivo anti-arterial thrombosis and thrombolytic activity, as well as downregulation of the expression of GPIIb/IIIa and P-selectin. (A) Dose-dependently anti-arterial thrombosis activities of MTCA-KKV; (B) Thrombolytic activity of 0.01 μmol/kg MTCA-KKV; (C) GPIIb/IIIa expression of the rats treated with 0.01 μmol/kg MTCA-KKV; (D) P-selectin expression of the rats treated with 0.01 μmol/kg MTCA-KKV; n=12.
Abbreviations: NS, normal saline; MTCA, (1R,3S)-1-methyl-1,2,3,4-tetrahydro-β-carboline-3-carboxylic acid; MTCA-KKV, (1R,3S)-1-methyl-1,2,3,4-tetrahydro-β-carboline-3-carboxyl-Lys(Pro-Ala-Lys)-Arg-Gly-Asp-Val.
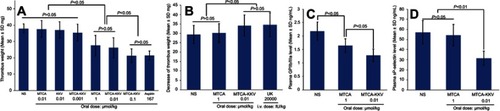
Figure 8 (A) ESI(+)-FT-MS spectrum of the extract of arterial thrombus of rats treated with 0.01 μmol/kg MTCA-KKV; (B) ESI(-)-FT-MS spectrum of the extract of arterial thrombus of rats treated with 0.01 μmol/kg of MTCA-KKV.
Abbreviations: MTCA-KKV, (1R,3S)-1-methyl-1,2,3,4-tetrahydro-β-carboline-3-carboxyl-Lys(Pro-Ala-Lys)-Arg-Gly-Asp-Val.
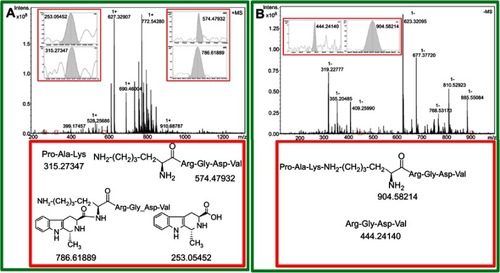
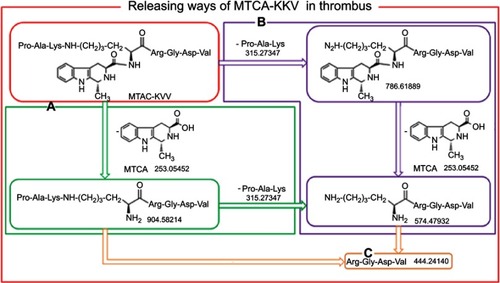
Figure 9 (A-C) In the arterial thrombus of the treated rats, MTCA-KKV is degraded via 3 ways to release anti-thrombosis, thrombolytic and thrombus targeting pharmacophores.
Abbreviations: MTCA, (1R,3S)-1-methyl-1,2,3,4-tetrahydro-β-carboline-3-carboxylic acid; MTCA-KKV, (1R,3S)-1-methyl-1,2,3,4-tetrahydro-β-carboline-3-carboxyl-Lys(Pro-Ala-Lys)-Arg-Gly-Asp-Val.