Figures & data
Figure 1 Schematic illustration of the conversion of (A) GO into (B) carboxyl-GO sheets via a facile one-step chloroacetic acid modification route at low temperature. (C) Carboxylic acid-terminated groups were formed and then activated with EDC/NHS to form an NHS ester-terminated group at the carboxyl-GO surface. (D) The attachment of peptides via amine coupling, and the deactivation of the unreacted surface sites with ethanolamine (EA) blocking. (E) Immobilization of the peptide on the carboxyl-GO-based SPR chip using non-immunological to detect hCG protein. (F) Schematic instrumental set-up of the Kretschmann configuration, the carboxyl-GO-peptide film was immobilized on the chip and injected into the hCG protein interaction of the SPR flow cell. The flow rate was 30 µL/minute, and the running buffer was phosphate-buffered saline (PBS) solution.
Abbreviations: GO, graphene oxide; hCG, human chorionic gonadotropin; SPR, surface plasmon resonance.
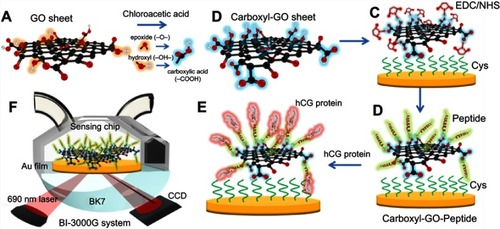
Figure 2 A scheme for the preparation of either functional carboxyl-GO modified (A) FTIR spectra, (B) Raman spectra, (C) SEM, and (D) TEM images.
Abbreviations: FTIR, Fourier transform infrared spectroscopy; SEM, scanning electron micrograph; STM, transmission electron microscopy.
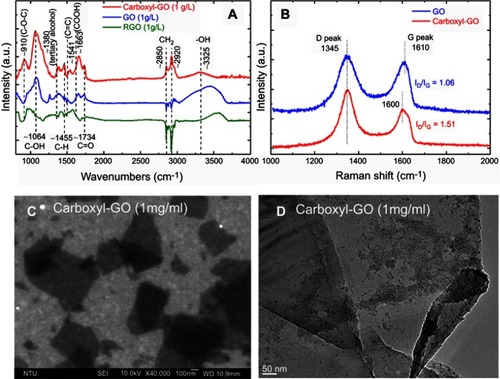
Figure 3 High resolution XPS spectra of the carboxyl-GO and GO films. (A) Survey scan XPS spectra of the different sensing chips. (B) N1s region for the as-formed carboxyl-GO/peptide chip. (C) C1s and (D) O1s regions for the as-formed carboxyl-GO chip. (E) C1s and (F) O1s regions for the as-formed GO film.
Abbreviations: XPS, X-ray photoelectron spectroscopy; GO, graphene oxide.
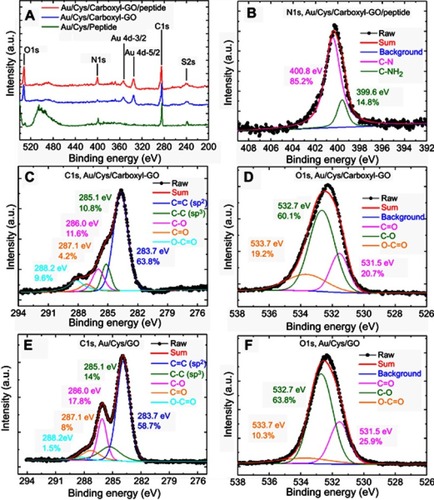
Figure 4 Cyclic voltammograms of bare Au, Au/Cys, Au/Cys/carboxyl-GO, and Au/Cys/carboxyl-GO/peptide chips at scan rates of (A) 10 mV/s and (B) 50 mV/s. (C) Cyclic voltammograms of the Au/Cys/carboxyl-GO/peptide chip with increases in scan rate from 10 to 50 mV/s. (D) The EIS spectra of the different sensing chips. (E) CV-SPR voltammograms of the different sensing chips at a potential scanning rate of 25 mV/s. (F) CV-SPR voltammograms of the Au/Cys/carboxyl-GO/peptide chip at different scan rates.
Abbreviations: Au, gold; Cys, cystamine; GO, graphene oxide; EIS, electrochemical impedance spectroscopy; CV, cyclic voltammetry; SPR, surface plasmon resonance.
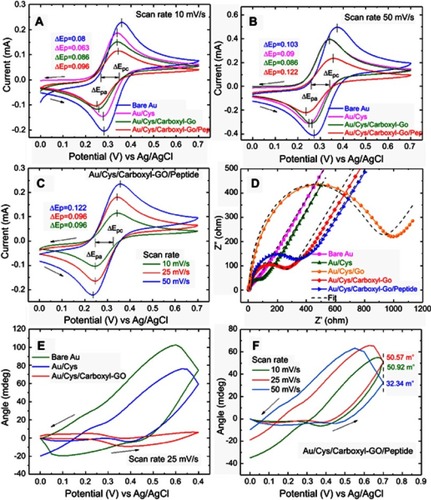
Figure 5 (A) The injection of peptides at a flow rate of 30 μL/min showing the three different SPR chip binding reactions. (B) The injection of peptides at different flow rates showing the carboxyl-GO ship binding reaction. (C) The binding kinetic constants at different flow rates in association and dissociation analyses.
Abbreviation: GO, graphene oxide.
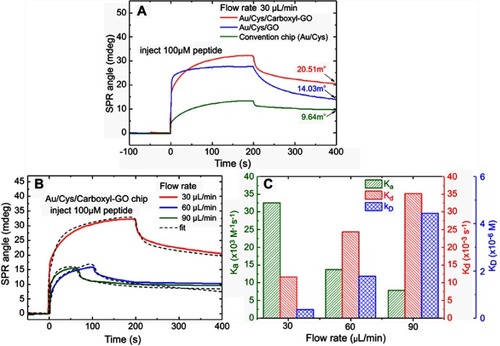
Figure 6 SPR sensorgrams for binding between peptides and hCG protein. The resonance angle shifts depended on (A) the carboxyl-GO chip with standard solutions of hCG concentrations alone, and (B) hCG mixed with BSA and HAS (20 nM each), respectively. (C) Characterization of peptide selectivity using both the single hCG protein and mixed protein experiments. (D) ka and kd values were obtained at different hCG protein concentrations by both kinetic and steady state fits.
Abbreviations: SPR, surface plasmon resonance; hCG, human chorionic gonadotropin; GO, graphene oxide; ka, association rate constant; kd, dissociation rate constant.
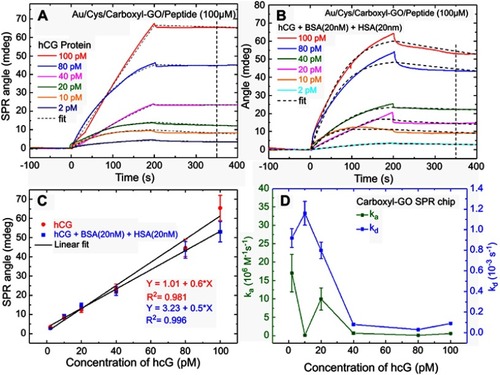
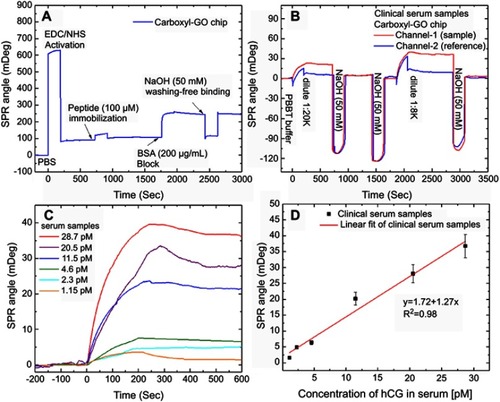
Figure 7 SPR sensorgrams of response curves for the assay of human hCG protein in the clinical serum samples. (A) SPR sensorgram of the peptide probe and BSA on the carboxyl-GO-based SPR aptasensor immobilization process steps of continuous reaction. (B) Sensorgram of two analysis cycles of a peptide–hCG protein interaction at different clinical serum sample concentrations. (C) The relationship between hCG protein and SPR angle shift was evaluated in serum samples with different dilutions. (D) Calibration plot for SPR angle shift with different dilutions of hCG serum samples at optimized conditions.
Abbreviations: SPR, surface plasmon resonance; hCG, human chorionic gonadotropin; GO, graphene oxide.