Figures & data
Figure 1 Schematic diagram of the LPHNPs.
Abbreviations: Doxo, Doxorubicin; DSPE-PEG 2000, 1,2-distearoyl-Sn-glycero-3-phosphoethanolamine-N-[methoxy (polyethylene glycol)]-2000; LPHNPs, lipid polymer hybrid nanoparticles; PLGA, poly (D, L-lactide-co-glicolide).
![Figure 1 Schematic diagram of the LPHNPs.Abbreviations: Doxo, Doxorubicin; DSPE-PEG 2000, 1,2-distearoyl-Sn-glycero-3-phosphoethanolamine-N-[methoxy (polyethylene glycol)]-2000; LPHNPs, lipid polymer hybrid nanoparticles; PLGA, poly (D, L-lactide-co-glicolide).](/cms/asset/007e0c68-fdc8-4e46-969f-fe8903bc161b/dijn_a_12190982_f0001_c.jpg)
Figure 2 TEM images of DOX.HCl (A and B), Blank LPHNPs (C and D), and DOX base (E and F) loaded LPHNPs.
Abbreviations: DOX, doxorubicin; DOX.HCl, doxorubicin hydrochloride; LPHNPs, lipid polymer hybrid nanoparticles; TEM, transmission electron microscopy.
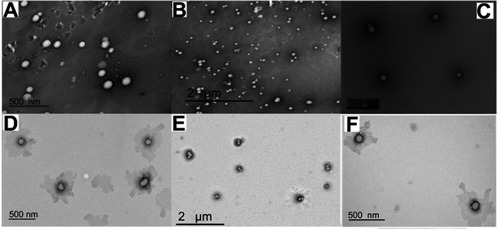
Table 1 Size, polydispersity (PDI) and zeta-potential of different prepared formulations
Table 2 Entrapment efficiency (EE) and percentage of drug release of the different prepared formulations
Table 3 Kinetic modeling of drug release of different prepared formulations after data fitting in different kinetic models
Figure 3 FTIR spectra of PLGA (A), lecithin (B), DSPE-PEG 2000 (C), Luterol (D), DOX (E) blank formulation (F), and DOX loaded LPHNPS (G).
Abbreviations: DOX, doxorubicin; DSPE-PEG 2000, 1,2-distearoyl-Sn-glycero-3-phosphoethanolamine-N-[methoxy (polyethylene glycol)]-2000; LPHNPs, lipid polymer hybrid nanoparticles; PLGA, poly (D, L-lactide-co-glicolide).
![Figure 3 FTIR spectra of PLGA (A), lecithin (B), DSPE-PEG 2000 (C), Luterol (D), DOX (E) blank formulation (F), and DOX loaded LPHNPS (G).Abbreviations: DOX, doxorubicin; DSPE-PEG 2000, 1,2-distearoyl-Sn-glycero-3-phosphoethanolamine-N-[methoxy (polyethylene glycol)]-2000; LPHNPs, lipid polymer hybrid nanoparticles; PLGA, poly (D, L-lactide-co-glicolide).](/cms/asset/a54c3ff3-f0a8-4b1a-b62e-018f96546ae9/dijn_a_12190982_f0003_c.jpg)
Figure 4 DSC thermograms of PLGA (A), lecithin (B), DSPE-PEG 2000 (C), physical mixture (D), DOX (E), blank LPHNPs (F) and DOX loaded LPHNPS (G).
Abbreviations: DOX, doxorubicin; DSC, differential scanning calorimetry; DSPE-PEG 2000, 1,2-distearoyl-Sn-glycero-3-phosphoethanolamine-N-[methoxy (polyethylene glycol)]-2000; LPHNPs, lipid polymer hybrid nanoparticles; PLGA, poly (D, L-lactide-co-glicolide).
![Figure 4 DSC thermograms of PLGA (A), lecithin (B), DSPE-PEG 2000 (C), physical mixture (D), DOX (E), blank LPHNPs (F) and DOX loaded LPHNPS (G).Abbreviations: DOX, doxorubicin; DSC, differential scanning calorimetry; DSPE-PEG 2000, 1,2-distearoyl-Sn-glycero-3-phosphoethanolamine-N-[methoxy (polyethylene glycol)]-2000; LPHNPs, lipid polymer hybrid nanoparticles; PLGA, poly (D, L-lactide-co-glicolide).](/cms/asset/e32e1a0d-a1b8-4d6f-bc15-e00c738a3671/dijn_a_12190982_f0004_c.jpg)
Figure 5 Colloidal stability studies, indicating the variation in particle size (A), PDI (B), and zeta-potential (C) in PBS, DMEM, and RPMI up to 4 hours at 37°C. All the results are presented in triplicate, along with error bars, as mean±SD (n=3).
Abbreviations: DMEM, Dulbecco’s Modified Eagle Medium; PBS, phosphate buffered saline; PDI, polydispersity; RPMI, Rosewell Park Memorial Institute.
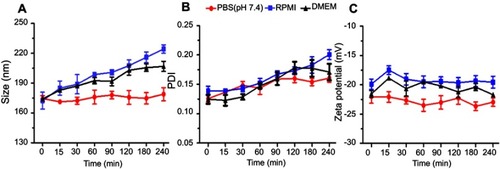
Figure 6 Cell viability study of the blank LPHNPs after 24 hours (A) and 48 hours (B) incubation with MDA-MB-231 cells at 37°C. The results are presented in triplicate with error bars as mean±SD (n=3).
Abbreviation: LPHNPs, lipid polymer hybrid nanoparticles.
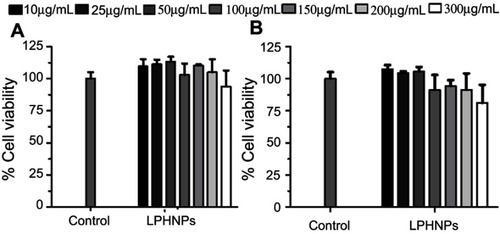
Figure 7 Cytotoxicity study of LPHNPs measured after 24 hours (A) and 48 hours (B) incubation with PC3 cells at 37°C. The results are presented in triplicate, with error bars as mean±SD (n=3).
Abbreviation: LPHNPs, lipid polymer hybrid nanoparticles.
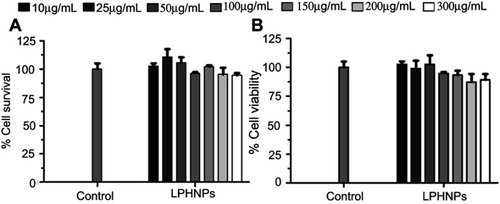
Figure 8 Confocal microscopic images of LPHNPs containing DOX solution. DOX.HCl and DOX base growing for 24 hours at 37°C in breast cancer cells. DAPI (blue) and CellMask Deep Red (red) were used to stain the different components of the cell. Green signals indicate the presence of DOX in the cells.
Abbreviations: DOX, doxorubicin; DOX-HCl, doxorubicin hydrochloride; LPHNPs, lipid polymer hybrid nanoparticles.
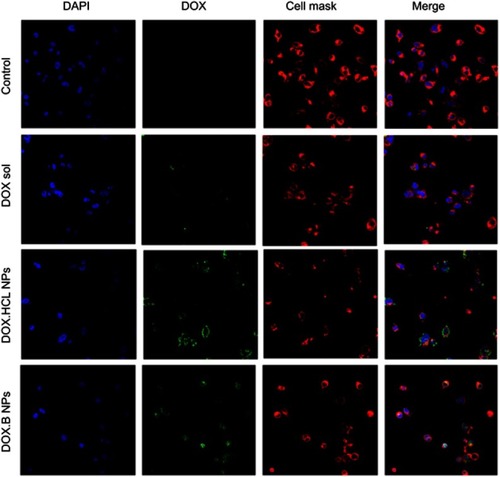
Figure 9 Cell growth inhibitory effects of the DOX solution, DOX.HCl loaded LPHNPs and DOX base loaded LPHNPs measured after 24 hours (A) and 48 hours (B) incubation along MDA-MB-231 cells. The activity was determined by CellTiter Glo viability assay. Results are presented with error bar, indicating mean±SD (n=3). *Significant where P>0.05, **significant where P>0.01.
Abbreviations: DOX, doxorubicin; DOX-HCl, doxorubicin hydrochloride; LPHNPs, lipid polymer hybrid nanoparticles; ns, non-significance.
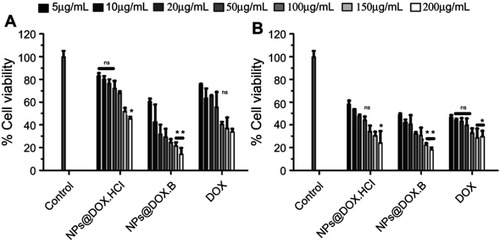
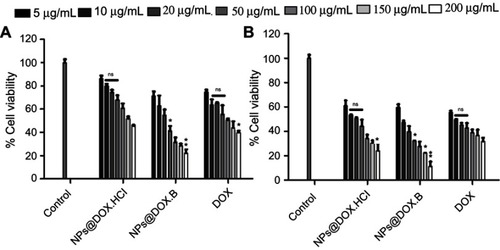
Figure 10 Cell growth inhibitory effects of the DOX solution, DOX.HCl loaded LPHNPs and DOX base loaded LPHNPs measured after 24 hours (A) and 48 hours (B) incubation along PC3 cells. The activity was determined by CellTiter Glo viability assay. Results are presented with error bar, indicating mean±SD (n=3). *Significant where P>0.05, **significant where P>0.01.
Abbreviations: DOX, doxorubicin; DOX-HCl, doxorubicin hydrochloride; LPHNPs, lipid polymer hybrid nanoparticles; ns, non-significance.