Figures & data
Figure 1 The mechanism of CCPM loading and releasing drugs by ion exchange.
Abbreviation: CCPM, carboxymethyl chitosan nanoporous microspheres.
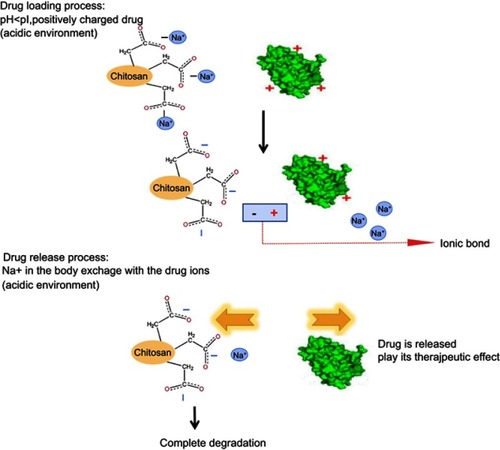
Figure 2 The diagram of the dynamic loading drug process.
Abbreviation: CCPM, carboxymethyl chitosan nanoporous microspheres.

Figure 3 Schematic diagram of the electrostatic self-assembly technique.
Abbreviations: rhIFNα-2b-CCPM, rhIFNα-2b carboxymethyl chitosan nanoporous microspheres; CS-rhIFNα-2b-CCPM, chitosan rhIFNα-2b carboxymethyl chitosan nanoporous microspheres.
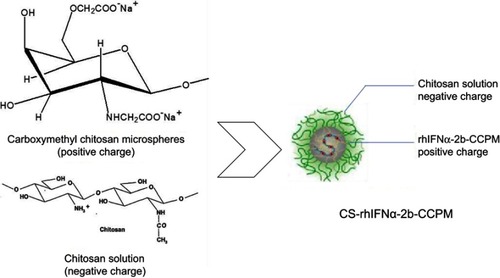
Figure 4 The effect of time on permeability and drug utilization rate of the drug-loading process (n=3).
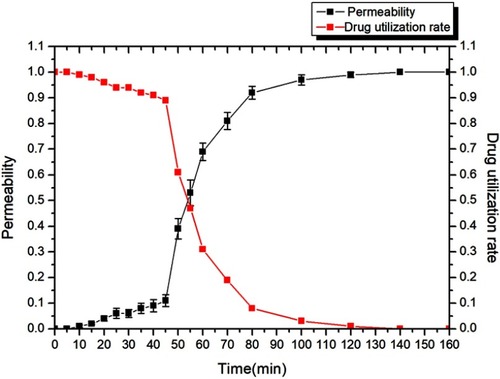
Figure 5 Characterization studies. (A) SEM images (ⅰ: CCPM; ⅱ: surface of CCPM; ⅲ: rhIFNα-2b-CCPM; ⅳ: CS-rhIFNα-2b-CCPM); (B) Particle size distribution of CS-rhIFNα-2b-CCPM; (C) Accumulative release from the optimal formulation; (D) Electrophoretogram of different rhIFNα-2b samples dyed by Coomassie brilliant blue (a: rhIFNα-2b solution; b: rhIFNα-2b extracted from nanoporous microspheres; c: rhIFNα-2b release solution for 12 hrs; d: rhIFNα-2b extracted from nanoporous microspheres after in vitro release for 24 hrs; e: rhIFNα-2b in effluent and washing liquid); (E) Circular dichroism spectra of rhIFNα-2b; (F) Inhibition rate of cell proliferation (n=3); and (G) Micrograph of the inhibition effect of the nanoporous microsphere releasing solution with different concentrations on A549 cells.
Abbreviations: CCPM, nanoporous microspheres; rhIFNα-2b-CCPM, rhIFNα-2b carboxymethyl chitosan nanoporous microspheres; CS-rhIFNα-2b-CCPM, chitosan rhIFNα-2b carboxymethyl chitosan nanoporous microspheres; IR, inhibition rate.
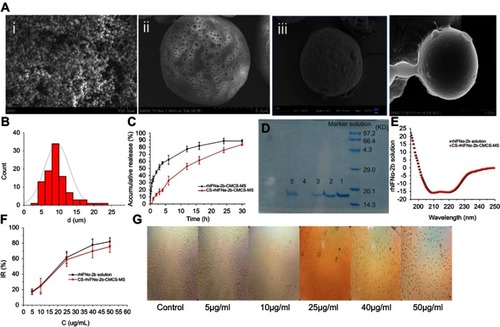
Table 1 Drug loading and encapsulation rate of the optimized prescription of CS-rhIFNα-2b-CCPM (n=3)
Table 2 Results of the drug release behavior fitted by different kinetic models. (mean±SD, n=6)
Table 3 Results of the recovery test (n=3)
Table 4 Pharmacokinetic parameters in mice plasma (mean±SD, n=3)
Figure 6 Drug concentration–time data following i.v. administration of the rhIFNα-2b solution and nanoporous microspheres in mice (n=3).
Abbreviation: CS-rhIFNα-2b-CMCS-MS, chitosan rhIFNα-2b carboxymethyl chitosan nanoporous microspheres.
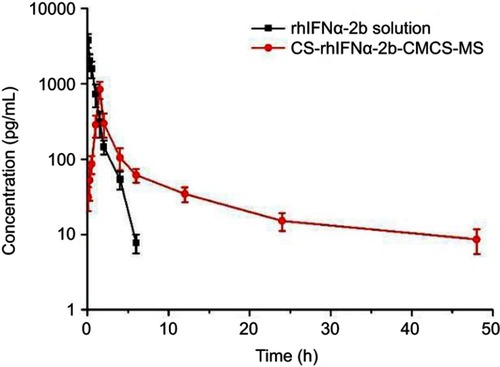
Figure 7 Tissue distribution diagram after tail vein injection (ng·g−1) (n=3). (A) histogram of drug concentration in each tissue after 0.5, 1, 4, and 6 hrs after rhIFNα-2b stock solution injection into the tail vein of mice. (B) Histogram of drug concentration in various tissues after the injection of rhIFNα-2b nanoporous microspheres into the tail vein of mice after 1, 4, 6, and 24 hrs.
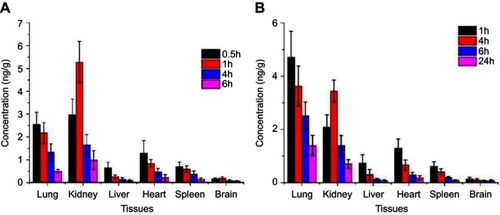
Table 5 Target index in mice tissues (mean ± SD, n=3)
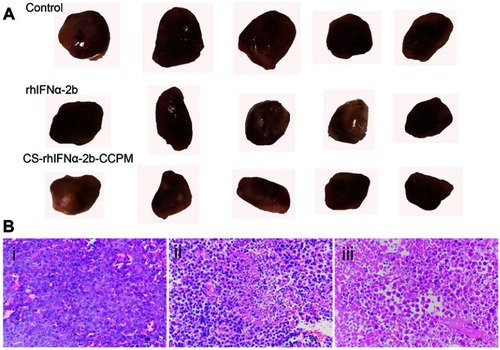
Figure 8 The evaluation of the antitumor effect (n=5). (A) The appearance of the tumors. (B) Histopathology slice of tumor (i: the control group; ii: the stock solution group; and iii: the microsphere group).
Abbreviation: CS-rhIFNα-2b-CCPM, chitosan rhIFNα-2b carboxymethyl chitosan nanoporous microspheres.