Figures & data
Table 1 Summarized Characteristics Of PMAA-AuNPs
Figure 1 Synthesis and characterization of PMAA-AuNPs. (A) Scheme of the direct synthesis of PMAA-AuNPs. (B) Cryo-TEM image of the obtained NPs. (C) UV-vis spectra of PMAA-AuNPs in various aqueous solvents (water, DPBS, DMEM, DMEM+10% FBS).
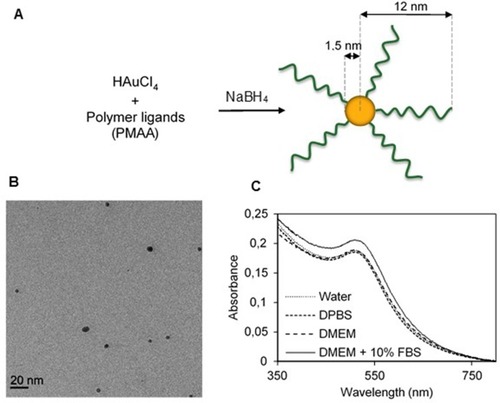
Figure 2 Sensitivity of NIS-expressing-B16 melanoma and -DHD colorectal carcinoma cells to radioiodine. (A) Immunofluorescence analysis of NIS expression in stably transfected B16-NIS cells. The expression of NIS at the cell surface was evaluated by using a specific anti‐mouse NIS polyclonal antibody (right panel) or rabbit IgG (left panel). (B) Cellular iodine uptake by the DHD-NIS and the B16-NIS tumor cells. DHD-NIS or B16-NIS cells were incubated for 1 hr with 125I in the presence or the absence of sodium perchlorate, a specific NIS inhibitor before being washed and lysed. Aliquots of lysates were counted in a γ counter. The data presented are the mean±standard error of the mean (SEM) of triplicates and are representative of three independent experiments. Radiation cell survival curves for DHD-NIS cells (C) and for B16-NIS cells (D). Clonogenic assays were performed on DHD-NIS or B16-NIS cells after treatment with 131I alone (0 to 11 MBq for 4 hrs) or with a combination of sodium perchlorate and 131I. ***p<0.001.
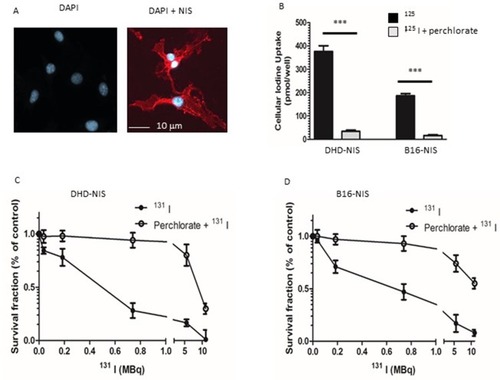
Figure 3 In vitro cytotoxicity and cellular uptake of PMAA-AuNPs nano-objects. Clonogenic assays were performed on DHD-NIS (A) or B16-NIS (B) cells treated for 2 hrs with PMAA-AuNPs nanoparticles at the indicated concentrations, before being washed and cultured for 9 to 14 days, respectively. The data presented are the mean±SEM of triplicates and are representative of three independent experiments. *p<0.05, **p<0.01, ***p<0.001. (C) Analysis of Au content per cell was conducted using a quadrupole ICP mass spectrometer, operated in standard mode. (*) indicates a significant difference observed in the Au uptake between the two cell types following exposure to the indicated concentrations of PMAA-AuNPs as determined by Student’s t test.
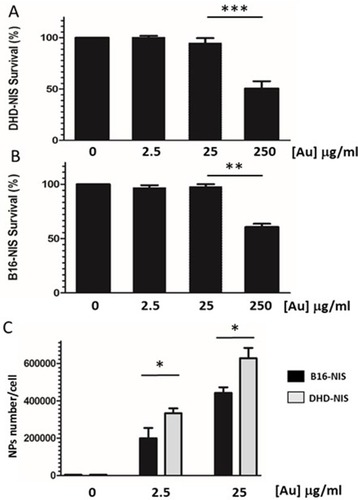
Figure 4 Radiosensitizing potential of PMAA-AuNPs to 131I on B16-NIS and DHD-NIS cells. Clonogenic assays were performed on DHD-NIS (A) or B16-NIS (B) cells treated for 2 hrs with PMAA-AuNPs nanoparticles at the indicated concentrations, before being washed and exposed to 131I alone (grey bars) (0.1 MBq for DHD-NIS cells, 0.2 MBq for B16-NIS cells) or to 131I in association with sodium perchlorate (black bars). The data presented are the mean±SEM of triplicates and are representative of three independent experiments. *p<0.05.
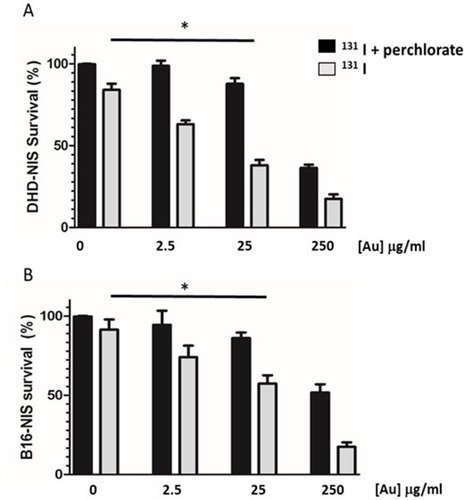
Figure 5 Assessment of radioiodine uptake by the NIS-expressing xenografts using microSPECT-CT imaging. (A) Radiotracer uptake was observed in tissues which express endogenous NIS in a control Balb/c female athymic mouse injected with an intraperitoneal administration of 15 MBq 99mTcO4- and imaged with a microSPECT-CT camera (eXplore speCZT, General Electric): thyroid (Th), salivary glands (SG), stomach (S), and urinary bladder (B). Representative SPECT/CT sagittal (B) and transverse (C) sections of three-dimensional images of a B16-NIS-bearing mouse 10 days after subcutaneous injection of the tumour cells. (T) B16-NIS tumor. (D) Hematoxylin-stained sections of B16-NIS tumors show highly proliferating cells. (E) Representative NIS staining of tumor sections shows heterogeneity in NIS expression levels within lesions.
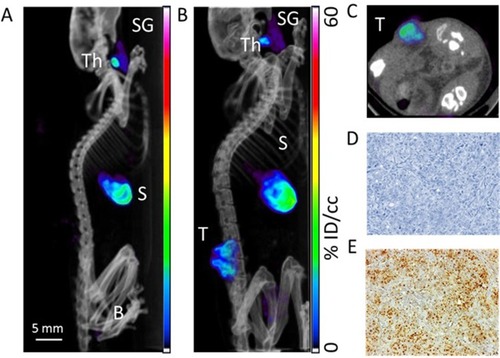
Figure 6 Assessment of radiosensitizing potential of PMAA-AuNPs to 131I in vivo. (A) Tumor development was monitored over time in mice untreated (filled circles) or treated with either PMAA-AuNPs alone (open circles), or with an intraperitoneal injection of 22 MBq 131 I (filled triangles), or with a combination of Au-PMAA/131 I (filled squares). (B) Tumor weight was evaluated at necropsy. (C) Representative photographs of tumor xenografts from mice treated with PMAA-AuNPs, with radioiodine, or with PMAA-AuNPs/radioiodine. (n=5 mice/condition). *p<0.05; **p<0.001.
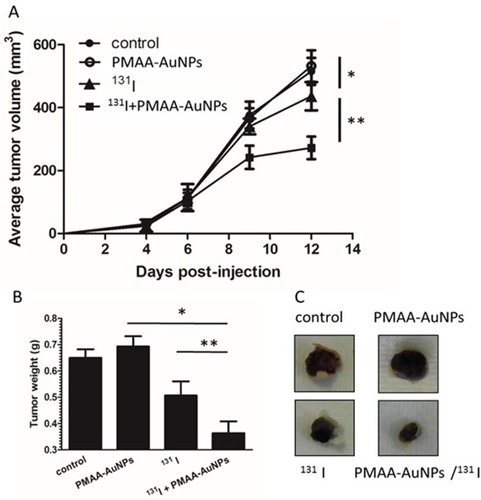
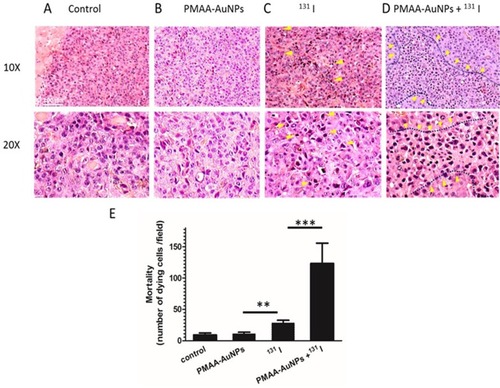
Figure 7 Histology of B16-NIS tumors after treatment with radioiodine combined or not with PMAA-AuNPs nanoparticles. Tumor histology was compared on sections (10× and 20× magnification) of lesions from B16-NIS challenged mice either untreated (A), or treated with PMAA-AuNPs (B), or with 20 MBq 131 I (C), or with PMAA-AuNPs/radioiodine (D). Arrows indicate the presence of pyknotic cells within lesions. (E) Numeration of the dying cells within lesions indicates a significantly increased mortality of the tumour cells in the PMAA-AuNPs/131I treated-lesions compared to all other groups of treatments. **p<0.001, ***p<0.0001.