Figures & data
Table 1 The formulations of two SMEDDS
Figure 1 The TEM pictures of BAPC-SMEDDS and CBA-SMEDDS.
Abbreviations: BAPC-SMEDDS, baicalein-phospholipid complex self-microemulsions; CBA-SMEDDS, conventional baicalein self-microemulsions.

Table 2 Characterization of BAPC-SMEDDS and CBA-SMEDDS (n=3)
Figure 2 The size distribution of BAPC-SMEDDS and CBA-SMEDDS, which were subjected to different folds of dilution with purified water (n=3).
Notes: A, dilution ratio (1:5–1:1000); B, dilution ratio (1:5–1:100).
Abbreviations: BAPC-SMEDDS, baicalein-phospholipid complex self-microemulsions; CBA-SMEDDS, conventional baicalein self-microemulsions.
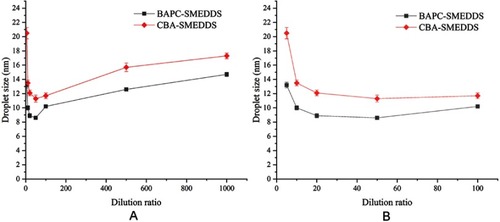
Figure 3 Delta transmission (△t) and turbiscan stability index (TSI) profiles of BAPC-SMEDDS and CBA-SMEDDS by using Turbiscan TOWER.
Abbreviations: BAPC-SMEDDS, baicalein-phospholipid complex self-microemulsions; CBA-SMEDDS, conventional baicalein self-microemulsions.
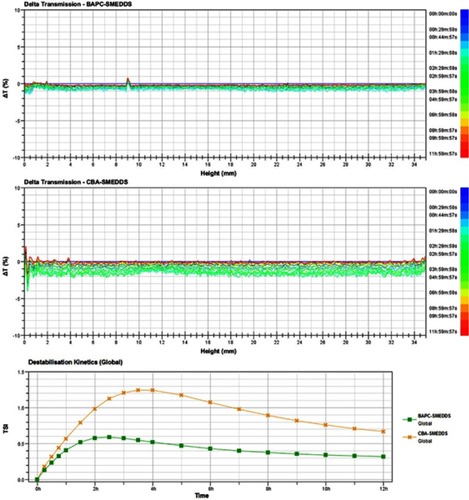
Figure 4 Release profile of baicalein from BAPC-SMEDDS, CBA-SMEDDS and BA (pH1.0, n=3).
Abbreviations: BAPC-SMEDDS, baicalein-phospholipid complex self-microemulsions; CBA-SMEDDS, conventional baicalein self-microemulsions; BA, free baicalein.
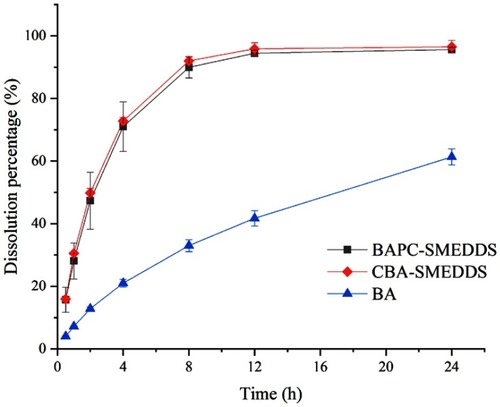
Figure 5 Release profile of baicalein from BAPC-SMEDDS, CBA-SMEDDS and BA (pH6.8, n=3).
Abbreviations: BAPC-SMEDDS, baicalein-phospholipid complex self-microemulsions; CBA-SMEDDS, conventional baicalein self-microemulsions; BA, free baicalein.
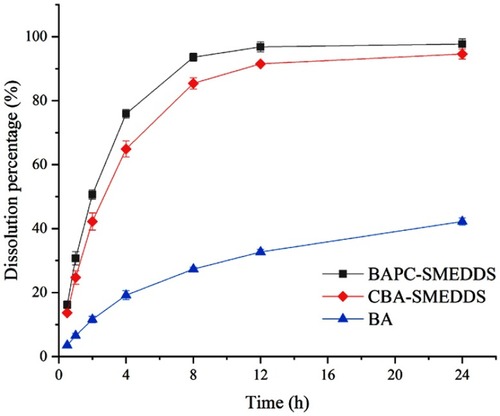
Figure 6 Consumption of 0.1 M NaOH during lipolysis of blank-SMEDDS with or without phospholipid (n=3).
Abbreviations: K-PC-SMEDDS, blank SMEDDS with phospholipid; K-SMEDDS, blank SMEDDS without phospholipid.
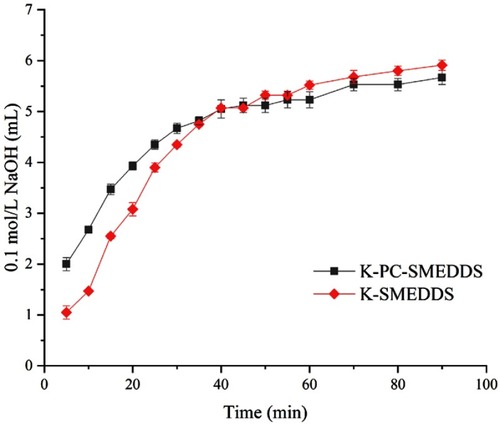
Figure 7 Mean droplet size of microemulsion globules or micelles formed during the in vitro lipolysis (n=3).
Abbreviations: BAPC-SMEDDS, baicalein-phospholipid complex self-microemulsions; CBA-SMEDDS, conventional baicalein self-microemulsions.
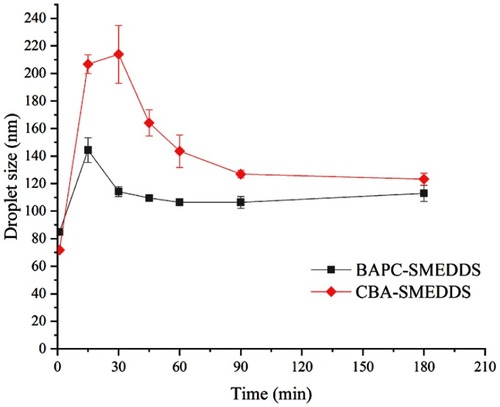
Figure 8 Distribution of baicalein in aqueous phase during lipolysis of BAPC-SMEDDS and CBA-SMEDDS (n=3).
Abbreviations: BAPC-SMEDDS, baicalein-phospholipid complex self-microemulsions; CBA-SMEDDS, conventional baicalein self-microemulsions.
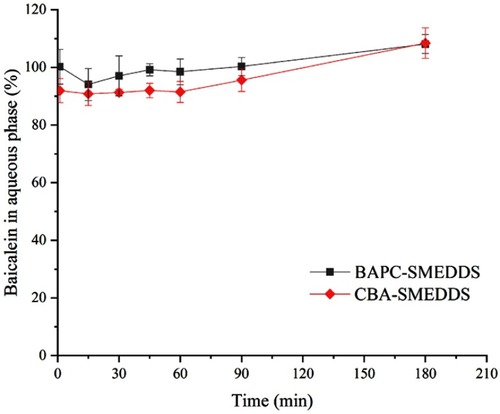
Table 3 Pharmacokinetic parameters of baicalin after oral administration of BAPC-SMEDDS, CBA-SMEDDS and BA (n=5)
Figure 9 Mean plasma concentration–time curves of baicalin in rats after oral administration of BAPC-SMEDDS, CBA-SMEDDS and BA (n=5).
Notes: The data were presented as mean ± SD (n=5).
Abbreviations: BAPC-SMEDDS, baicalein-phospholipid complex self-microemulsions; CBA-SMEDDS, conventional baicalein self-microemulsions; BA, free baicalein.
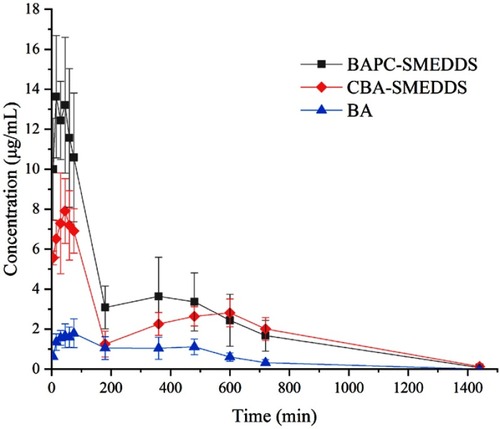
Figure 10 Mean plasma concentration–time curves of baicalein in rats after oral administration of BAPC-SMEDDS, CBA-SMEDDS and BA (n=5).
Notes: The data were presented as mean ± SD (n=5).
Abbreviations: BAPC-SMEDDS, baicalein-phospholipid complex self-microemulsions; CBA-SMEDDS, conventional baicalein self-microemulsions; BA, free baicalein.
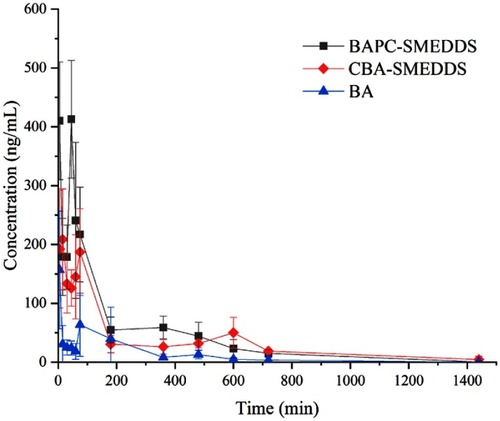
Figure 11 The average AUC0-t and Cmax of baicalein after oral administration of BAPC-SMEDDS, CBA-SMEDDS and BA (n=5).
Abbreviations: BAPC-SMEDDS, baicalein-phospholipid complex self-microemulsions; CBA-SMEDDS, conventional baicalein self-microemulsions; BA, free baicalein; AUC, area under the curve; Cmax, peak concentration.
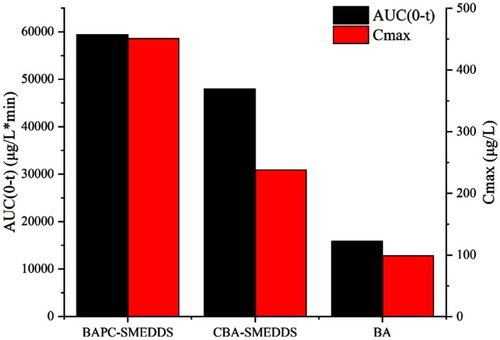
Table 4 Pharmacokinetic parameters of baicalin within 3 hrs after oral administration of BAPC-SMEDDS, CBA-SMEDDS and BA (n=5)
Table 5 Pharmacokinetic parameters of baicalin within 3 hrs after intraperitoneal pretreatment with 3.0 mg/kg cycloheximide following oral administration of BAPC-SMEDDS, CBA-SMEDDS and BA (n=5)
Table 6 Fraction of baicalein transported directly to the systemic blood circulation and fraction of baicalein transported indirectly to the systemic blood circulation via intestinal lymphatic system after oral administration of baicalein in different formulations to rats
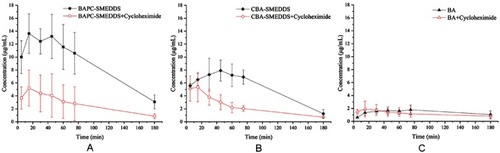
Figure 12 Mean plasma concentration–time curves of baicalin in rats within 3 hrs after intraperitoneal pretreatment with or without 3.0 mg/kg cycloheximide following oral administration of BAPC-SMEDDS, CBA-SMEDDS and BA (n=5).
Notes: A, intraperitoneal pretreatment with or without 3.0 mg/kg cycloheximide following oral administration of BAPC-SMEDDS; B, intraperitoneal pretreatment with or without 3.0 mg/kg cycloheximide following oral administration of CBA-SMEDDS; C, intraperitoneal pretreatment with or without 3.0 mg/kg cycloheximide following oral administration of BA. The data were presented as mean ± SD (n=5).
Abbreviations: BAPC-SMEDDS, baicalein-phospholipid complex self-microemulsions; CBA-SMEDDS, conventional baicalein self-microemulsions; BA, free baicalein.