Figures & data
Table 1 Optimization Of PEI Ratio In W Phase On eBev-DPPNs
Table 2 Optimization Of The Ratio Of DPPNs And Bevacizumab On eBev-DPPNs
Table 3 Characteristics Of The Optimized Formulations
Figure 1 Characteristics of the eBev-DPPNs and cBev-DPNs. (A) Size distribution of eBev-DPPNs based on DLS; (B) Size distribution of cBev-DPNs based on DLS; (C) SEM images of eBev-DPPNs; (D) SEM images of cBev-DPNs. Data are expressed as mean ± SD, n = 3.
Abbreviations: eBev-DPPNs, electrostatically-conjugated bevacizumab-bearing DPPNs; cBev-DPNs, chemically-conjugated bevacizumab-bearing DPNs; DLS, dynamic light scattering; SEM, scanning electron microscopy.
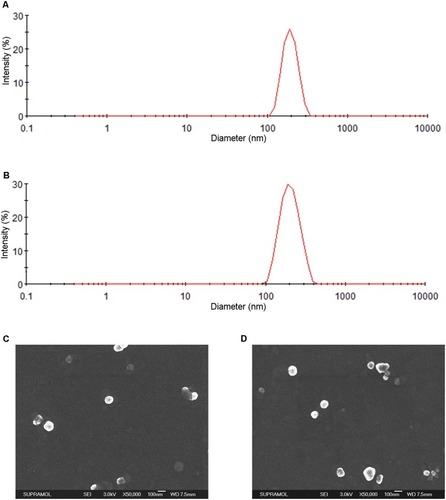
Figure 2 Stability of the eBev-DPPNs and cBev-DPNs. (A) Particle size; (B) PDI; (C) Binding efficiency of bevacizumab in PBS; (D) Particle size; (E) PDI; (F) Binding efficiency of bevacizumab in the vitreous humor of rabbit eyes. Data are expressed as mean ± SD, n = 3.
Abbreviations: eBev-DPPNs, electrostatically-conjugated bevacizumab-bearing DPPNs; cBev-DPNs, chemically-conjugated bevacizumab-bearing DPNs.
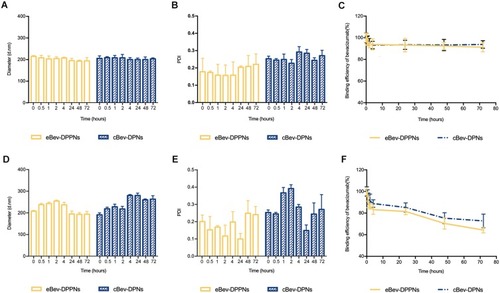
Figure 3 Structural stability of the bevacizumab on the surface of the eBev-DPPNs and cBev-DPNs.
Notes: (A) SEC-HPLC chromatograms; (B) CD spectra; (C) Fluorescence spectra.
Abbreviations: eBev-DPPNs, electrostatically-conjugated bevacizumab-bearing DPPNs; cBev-DPNs, chemically-conjugated bevacizumab-bearing DPNs.
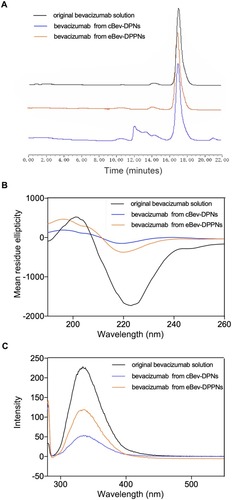
Figure 4 (A) In vitro release of dexamethasone from the eBev-DPPNs and cBev-DPNs; (B) In vitro release of bevacizumab from the eBev-DPPNs and cBev-DPNs. Data are expressed as mean ± SD, n = 3.
Abbreviations: eBev-DPPNs, electrostatically-conjugated bevacizumab-bearing DPPNs; cBev-DPNs, chemically-conjugated bevacizumab-bearing DPNs.
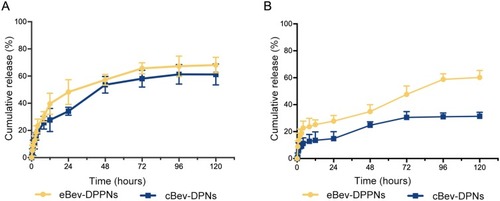
Figure 5 Induction of apoptosis by nanoparticles on HUVECs. Data are expressed as mean ± SD, n = 6.
Abbreviations: eBev-DPPNs, electrostatically-conjugated bevacizumab-bearing DPPNs; cBev-DPNs, chemically-conjugated bevacizumab-bearing DPNs; PI, propidium iodide.
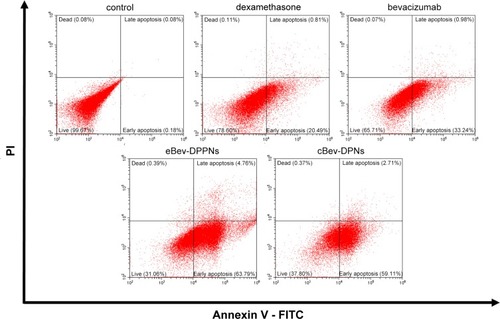
Figure 6 Migration inhibition by nanoparticles on HUVECs in wound healing assays. (A) Representative images of HUVECs migration; (B) Migration number of HUVECs. Data are expressed as mean ± SD, n = 6. **p < 0.01, ***p < 0.001 vs control; #p < 0.05, ##p < 0.01, ###p < 0.001 vs eBev-DPPNs.
Abbreviations: eBev-DPPNs, electrostatically-conjugated bevacizumab-bearing DPPNs; cBev-DPNs, chemically-conjugated bevacizumab-bearing DPNs.
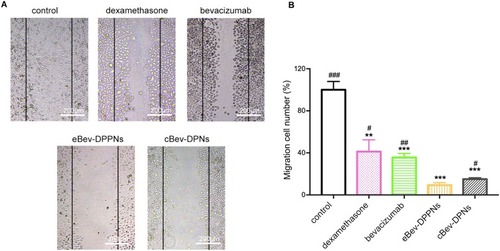
Figure 7 Invasion inhibition by nanoparticles on HUVECs in Transwell assays. (A) Representative images of HUVECs invasion; (B) Invasion number of HUVECs. Data are expressed as mean ± SD, n = 6. **p < 0.01, ***p < 0.001 vs control; #p < 0.05, ###p < 0.001 vs eBev-DPPNs.
Abbreviations: eBev-DPPNs, electrostatically-conjugated bevacizumab-bearing DPPNs; cBev-DPNs, chemically-conjugated bevacizumab-bearing DPNs.
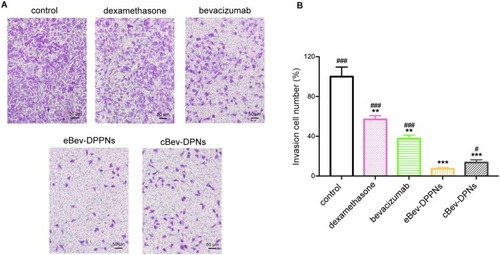
Figure 8 Tube formation inhibition by nanoparticles on HUVECs. (A) Representative images of HUVECs tube formation; (B) Tube formation capacity of HUVECs. Data are expressed as mean ± SD, n = 6. **p < 0.01, ***p < 0.001 vs control; ##p < 0.01, ###p < 0.001 vs eBev-DPPNs.
Abbreviations: eBev-DPPNs, electrostatically-conjugated bevacizumab-bearing DPPNs; cBev-DPNs, chemically-conjugated bevacizumab-bearing DPNs.
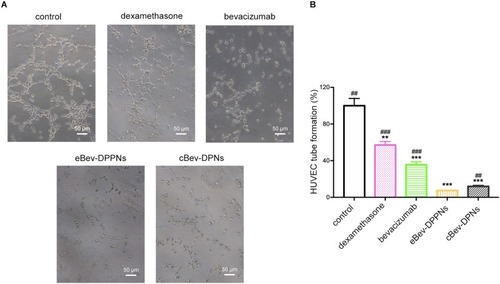
Figure 9 VEGF secretion inhibition by nanoparticles on HUVECs. (A) VEGF secretion at 12 h; (B) VEGF secretion at 24 h; (C) VEGF secretion at 48 h; (D) VEGF secretion at 72 h. Data are expressed as mean ± SD, n = 6. *p < 0.05, **p < 0.01 vs control without CoCl2; #p < 0.05, ##p < 0.01, ###p < 0.001 vs control with CoCl2; ^p < 0.05, ^^p < 0.01 vs eBev-DPPNs with CoCl2.
Abbreviations: VEGF, vascular endothelial growth factor; eBev-DPPNs, electrostatically-conjugated bevacizumab-bearing DPPNs; cBev-DPNs, chemically-conjugated bevacizumab-bearing DPNs.
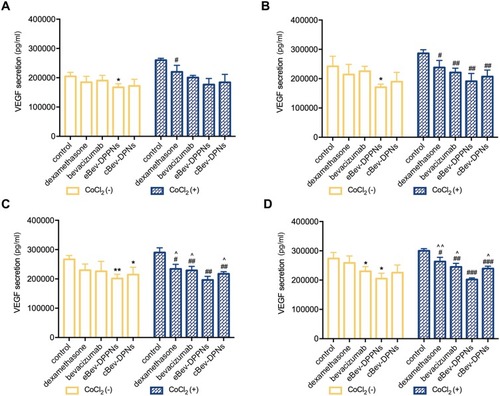
Figure 10 Anti-angiogenesis effects in vivo on chorioallantoic membranes. (A) Representative images of angiogenesis; (B) The amount of blood vessel on chorioallantoic membranes. Data are expressed as mean ± SD, n = 10. **p < 0.01, ***p < 0.001 vs control; #p < 0.05, ##p < 0.01, ###p < 0.001 vs eBev-DPPNs.
Abbreviations: eBev-DPPNs, electrostatically-conjugated bevacizumab-bearing DPPNs; cBev-DPNs, chemically-conjugated bevacizumab-bearing DPNs.
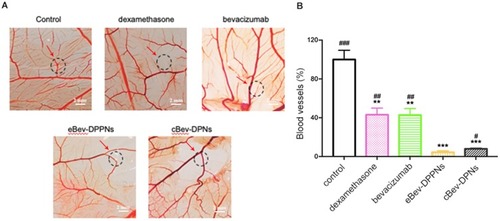
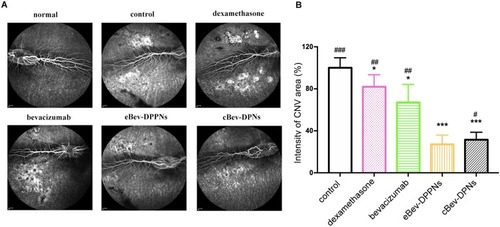
Figure 11 The inhibition of CNV area on laser-induced CNV rabbits model. (A) Representative images of the leakage area of CNV; (B) The intensity of CNV area on rabbits fundus. Data are expressed as mean ± SD, n = 3. *p < 0.05, ***p < 0.001 vs control; #p < 0.05, ##p < 0.01, ###p < 0.001 vs eBev-DPPNs.
Abbreviations: eBev-DPPNs, electrostatically-conjugated bevacizumab-bearing DPPNs; cBev-DPNs, chemically-conjugated bevacizumab-bearing DPN; CNV, Choroidal neovascularization.