Figures & data
Figure 1 The structure of blood–brain barrier (BBB) and blood–brain tumor barrier (BBTB). The blood vessels with the intact BBB are enclosed by endothelial cells with tight junction while the tight junction between endothelial cells is disrupted by infiltration of tumor cells in the BBTB.
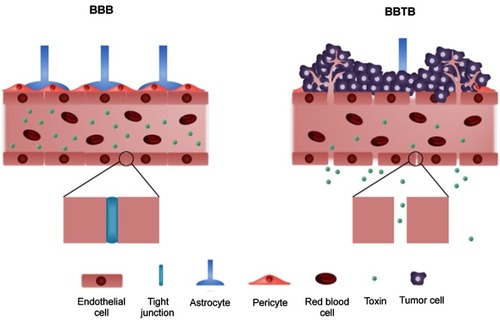
Figure 2 The radiotherapy resistance mechanism in glioma cell. After the break of DNA, Ku70/Ku80 heterodimers bind to damaged ends of DNA and triggers the recruitment of overexpressed DNA-PKcs from EGFRvIII to the damaged DNA ends. Then, XRCC4, DNA ligase IV and XLF subsequently bind to the damaged ends of DNA for the ligation.
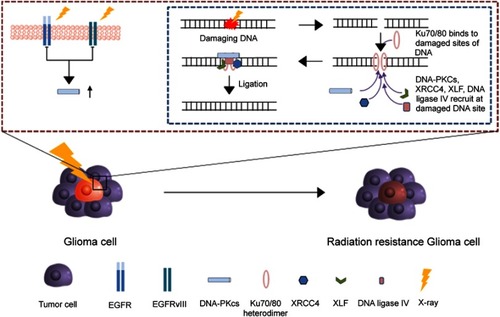
Figure 3 The tumor tropism of mesenchymal stem cells (MSCs). (A) Schematic illustration of tumor tropism of CXCR4-overexpressed MSCs (B) Homing mechanism of MSCs to tumor sites.
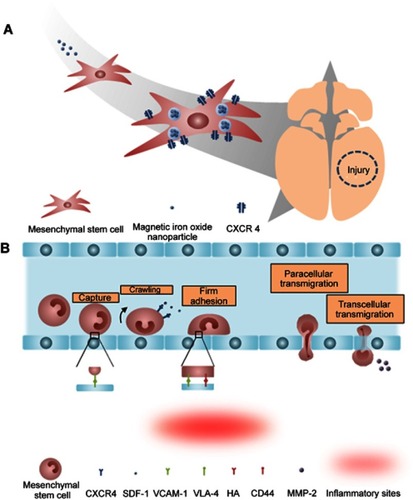
Figure 4 Tumor anti-angiogenesis induced by mesenchymal stem cell (MSC). (A) The gross image and histological analysis of Gil36 and Gil36/MSC co-cultured tumor section (B) Scheme of anti-angiogenesis of MSC when co-cultured with Gli36 cell (C) Schematic figure of capillary degeneration induced by connexin-43 gap junctional channel between MSC and endothelial cell. Reproduced from Ho IA, Toh HC, Ng WH, et al. Human bone marrow-derived mesenchymal stem cells suppress human glioma growth through inhibition of angiogenesis. Stem Cells. 2013;31(1):146–155, with permission from John Wiley and Sons.Citation106
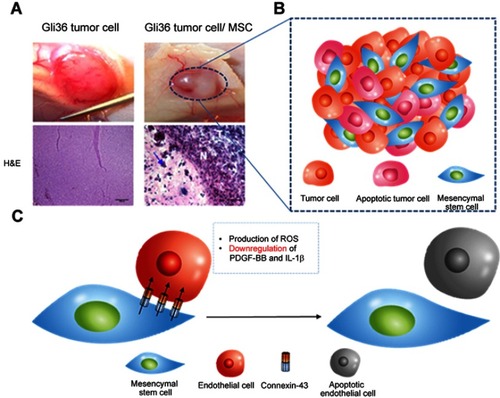
Figure 5 The mechanism of oncolytic virus-loaded mesenchymal stem cell therapy. Replication of oncolytic virus in healthy cells is inhibited and is induced in tumor cells. The rapid replication of oncolytic virus in tumor cells triggers the apoptosis of tumor cells and infects neighboring tumor cells.
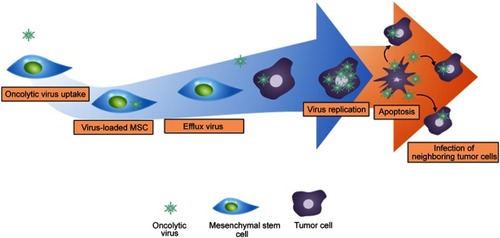
Figure 6 Mesenchymal stem cell (MSC) as a chemotherapeutic carrier. (A) Lung tumor homing ability of MSCs (B) Schematic figure of CD73 or CD90 conjugation to MSCs (C) Schematic figure of silica nanorattle conjugated MSC in xenograft model and nanodrug conjugated MSC for A549 deep tumor treatment. Reproduced from Kim SW, Lee YK, Hong JH, et al. Mutual destruction of deep lung tumor tissues by nanodrug-conjugated stealth mesenchymal stem cells. Adv Sci (Weinh). 2018;5(5):1700860.Citation24
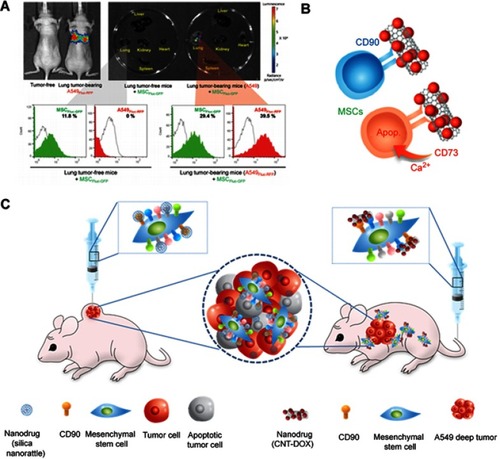
Figure 7 The anti-inflammatory mechanism of mesenchymal stem cells (MSCs). Anti-inflammatory cytokines released from MSCs suppress M1 polarization while induce M2 polarization. M2 polarization subsequently triggers the proliferation of regulatory T cells. The red arrow represents suppression while black arrow refers to promotion of the process.
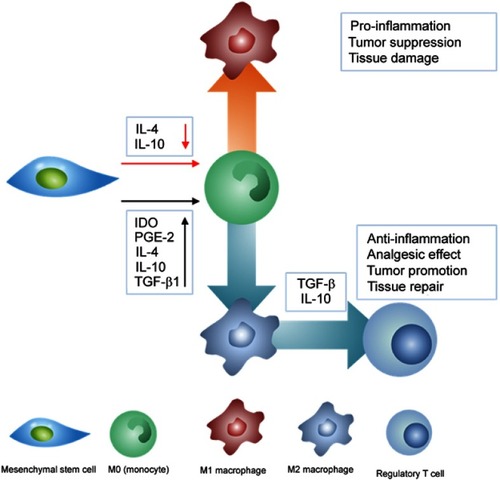
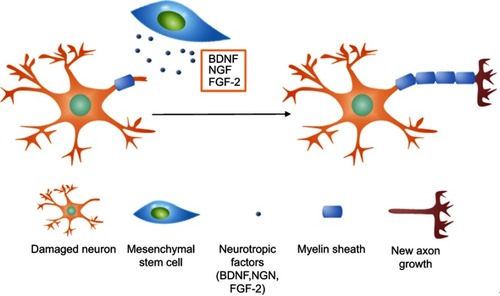
Figure 8 The mechanism of nerve regeneration assisted by mesenchymal stem cells. Neurotrophic factors including BDNF, NGF, and FGF-2 released by MSCs interact with damaged axons and induce axonal outgrowth.