Figures & data
Figure 1 Biofilm pathogenicity in humans is mediated by dissemination and biofilm matrix formation. (A) Possible routes of biofilm dissemination around the body originating from sites such as gum disease, catheter, or implant contamination.Citation12 (B) Scanning electron micrographs (SEM) of biofilm matrix, offering protection of resident bacteria from eradication, imaged from a clinical endotracheal tube identified as Streptococcus pneumonia. Scale bar shown is 1 μm. (C) Schematic of biofilm-mediated device-related infection, starting with bacterial attachment to a device or adsorbed host proteins, leading to biopolymer mediated cell–cell adhesion, maturation, and eventual detachment leading to the spread of infection.Citation66
Note: A is reproduced from Hall-Stoodley et alCitation12 and C is reproduced from OttoCitation66 with permission from the publishers.
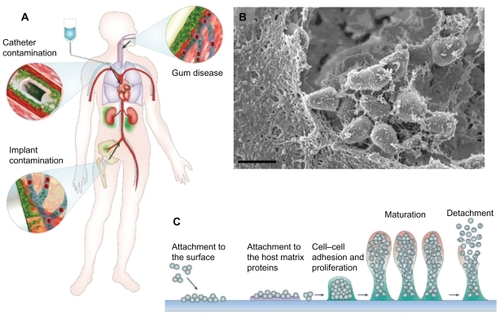
Figure 2 Various nanomaterials are being studied for emergent antimicrobial properties and are being used for the design of the next generation of therapeutics and biomaterials. Zinc oxide nanoparticles (shown in A) have a high surface area and a particle size of about 12 nm, demonstrating antibacterial activity (scanning electron micrograph; scale bar: 20 μm). Vancomycin-coated gold nanoparticles (shown in B), with an inset at the minimum inhibitory concentration, are able to overcome resistance in several strains of vancomycin-resistant bacteria (transmission electron micrograph; scale bar: 20 nm).Citation37 The enhanced antimicrobial properties of single-walled carbon nanotubes compared with larger diameter multiwalled carbon nanotubes (as shown in C and D, respectively) could be used as conductive biomaterials, in situ sensors, or tissue engineering scaffolds (scale bars: 2 μm and 50 nm, as shown in inset).Citation54 Superparamagnetic iron oxide nanoparticles (shown in E; scale bar: 100 nm) with antimicrobial properties are being explored for controlled delivery to infection sites.
Notes: A is reprinted with permission from Raghupathi KR, Koodali RT, Manna AC. Size-dependent bacterial growth inhibition and mechanism of antibacterial activity of zinc oxide nanoparticles. Langmuir. 2011;27(7):4020–4028. Copyright 2011 American Chemical Society. B is reprinted with permission from Gu H, Ho PL, Tong E, Wang L, Xu B. Presenting vancomycin on nanoparticles to enhance antimicrobial activities. Nano Letters. 2003;3(9):1261–1263. Copyright 2003 American Chemical Society. C and D are reprinted with permission from Kang S, Mauter MS, Elimelech M. Microbial cytotoxicity of carbon-based nanomaterials: implications for river water and wastewater effluent. Environ Sci Technol. 2009;43(7):2648–2653. Copyright 2009 American Chemical Society.
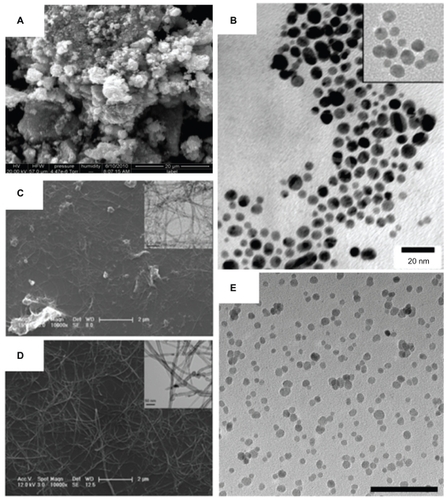
Figure 3 Transmission electron micrographs of Staphylococcus aureus bacteria interacting with nanoparticles including superparamagnetic iron oxide nanoparticles (A and D), cowpea chlorotic mottle virus (CCMV) nanoparticles (B and E), and self-assembled cationic peptide nanoparticles (C and F). Magnetic nanoparticles bind to the bacterial cell surface resulting in membrane disruption (A) and nanoparticle penetration (D) potentially used for bacterial separations. CCMV binds to a coating of protein A antibodies on S. aureus membranes through biotin–streptavidin interactions (B) without penetration (E; inset: close-up view of the coated surface) for targeting of magnetic resonance imaging contrast agents.Citation40 S. aureus before (C) and after (F) membrane disruption by self-assembled cationic peptide nanoparticles, leading to a rough surface and the formation of a minicell.Citation41
Note: Scale bars: A, C–F, 100 nm; B, 200 nm. B and E are reprinted from Chemistry and Biology, 14/4, Suci PA, Berglund DL, Liepold L, et al, High-density targeting of a viral multifunctional nanoplatform to a pathogenic, biofilm-forming bacterium, 387–398, 2007, with permission from Elsevier. C and F are reproduced from Liu et al with permission from the publisher.Citation41
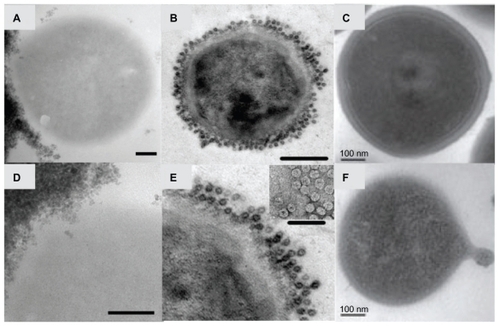
Figure 4 Staphylococcus aureus biofilm penetration by cowpea chlorotic mottle virus (CCMV) (A) and superparamagnetic iron oxide nanoparticles (SPION) (B), analyzed by confocal fluorescence microscopy and Prussian blue histology stains for iron, respectively. (A) S. aureus biofilm penetrated by a CCMV nanoplatform (green fluorescence in micrograph) demonstrating a penetration depth of 17.6 μm during an 80-minute exposure (main panel shows the top view of the biofilm, and the right and bottom panels are cross-sectional views showing CCMV penetration depth)Citation40 (scale bar: 30 μm). (B) Bulk penetration of SPION into an S. aureus biofilm cannot be observed without magnetic field exposure for 1 hour (magnetic field time zero; t = 0), and is enhanced through application of a magnetic field (magnetic field time 20, 40, or 60 minutes; t = 20, 40, or 60).
Note: A is reprinted from Chemistry and Biology, 14/4, Suci PA, Berglund DL, Liepold L, et al, High-density targeting of a viral multifunctional nanoplatform to a pathogenic, biofilm-forming bacterium, 387–398, 2007, with permission from Elsevier.
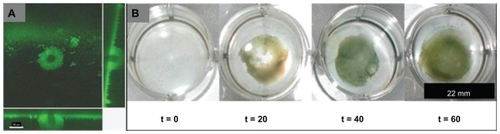
Figure 5 The diameter of the carbon nanotubes determined by transmission electron microscopy (A and C) predicts antimicrobial behavior as visible in scanning electron micrographs (B and D).Citation53 A mat-like surface made of multiwalled carbon nanotubes (A) inactivates a small percentage of Escherichia coli, visible as abnormal and flattened bacteria on the surface (B). Single-walled carbon nanotubes (C), having a smaller diameter, inactivate a greater percentage of bacteria, causing significant structural disruption and bacterial death (D).
Note: Reprinted with permission from Kang S, Mauter MS, Elimelech M. Microbial cytotoxicity of carbon-based nanomaterials: implications for river water and wastewater effluent. Environ Sci Technol. 2009;43(7):2648–2653. Copyright 2009 American Chemical Society.
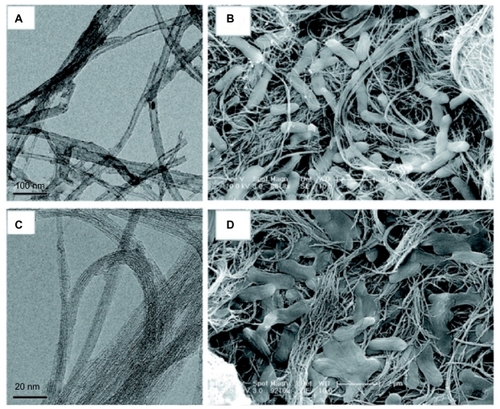
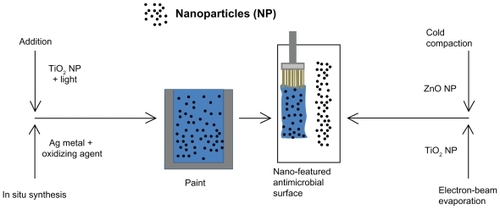
Figure 6 Processes for the creation of nano-featured antimicrobial surfaces using addition,Citation59 in situ synthesis,Citation60 cold compation,Citation49 or electron-beam evaporationCitation48 incorporating nanoparticles. During addition, nanoparticles are simply added to the paint and, in the case of TiO2 nanoparticles, are activated using a light source, such as a fluorescent light.Citation59 In situ synthesis uses the properties of the paint to reduce silver (Ag) metal into nanoparticles, for example using a metal-catalyzed free-radical-mediated oxidation process during the production of silver nanoparticles in vegetable oil–based paints.Citation60 During cold compaction, nanoparticles are pressurized into a nano-featured surface, as previously achieved using a simple uniaxial, single-ended compacting hydraulic press held at 275 MPa for 30 seconds, then held at a final pressure of 550 MPa for 1 minute before releasing the pressure completely.Citation49 Electron-beam evaporation concentrates a large amount of heat produced by high-energy electron-beam bombardment on the source material to be deposited, in this case pure Ti pellets, whereby heating and vaporization occur.Citation48 The vapor flow then condenses onto the substrate surface located at the top of the vacuum chamber to control nano-featured or larger surface-feature creation.Citation48