Figures & data
Figure 1 Production variables known to influence extracellular vesicle (EV) properties such as the cell type, cellular origin, cell culture conditions, mechanisms used to trigger EV release, isolation procedure of EVs, and storage conditions.
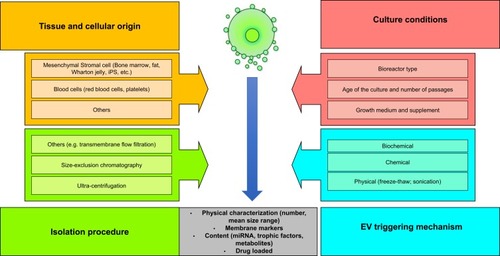
Figure 2 Illustration of manufacturing approaches considered for the production of extracellular vesicle (EV)-based therapeutics under good manufacturing practice-compliant, and scalable downstream processing methods to ensure the quality, safety, and consistency. Reprinted from of Trends Biotechnol. 37. Agrahari V, Agrahari V, Burnouf PA, Chew CH, Burnouf T. Extracellular microvesicles as new industrial therapeutic frontiers. 707–729. Copyright (2019).Citation3 with permission from Elsevier.
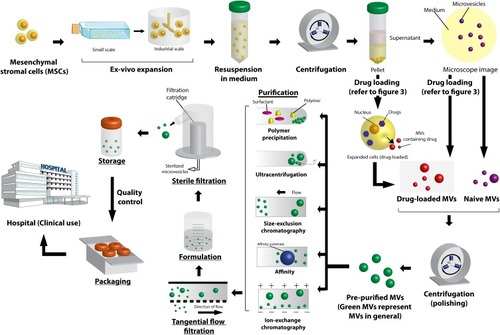
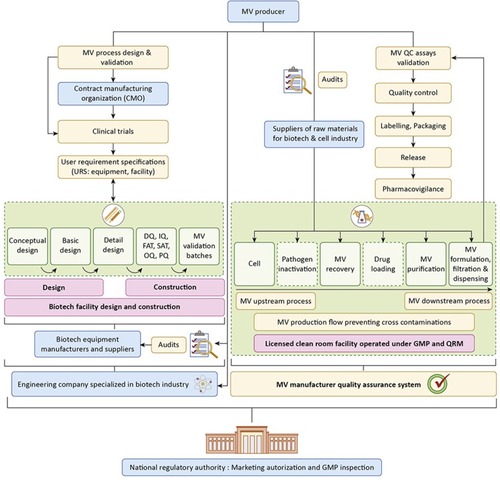
Figure 3 Phase-by-phase proposed development of an industrial microvesicle (MV) project and main stakeholders (identified in boxes with a light-blue background). After conducting pilot-scale design and validation of the MV production process at the research and development scale, the MV producer engages a contract manufacturing organization to produce MV batches for clinical trials under investigational new drug status, or equivalent, under supervision of a competent National Regulatory Authority (NRA). Once clinical data are conclusive, the MV producer hires an engineering company specializing in the biotechnology industry, which by closely following the user requirement specifications from the MV producer, can proceed with the conceptual, basic, and detailed design phases of the facility and select suitable equipment from qualified biotechnology industry suppliers. Next, the construction of the facility and the making of equipment go through the qualification phases: design qualification (DQ), installation qualification (IQ), factory acceptance tests (FAT), site acceptance tests (SATs), operational qualification (OQ), performance qualification (PQ), and production of consecutive MV validation batches meeting pre-established quality specifications. Following submission and approval of all the required validation and clinical documentation, the manufacturing site is licensed, and the MV product receives a marketing authorization by the relevant NRA. The MV producer operates the facility under good manufacturing practices (GMPs) and conducts quality audits of the suppliers of raw materials (eg, growth medium; fetal bovine serum, and buffer components) and excipients used during manufacture. The facility is operated under GMPs and is subjected to periodic NRA inspections to maintain manufacturing site license and MV marketing authorization. The MV product is subject to pharmacovigilance, in particular to monitor adverse reactions. Reprinted from of Trends Biotechnol. 37. Agrahari V, Agrahari V, Burnouf PA, Chew CH, Burnouf T. Extracellular microvesicles as new industrial therapeutic frontiers. 707–729. Copyright (2019)Citation3. With permission from Elsevier.
Abbreviation: QRM, quality risk management.