Figures & data
Table 1 The mean particle size and zeta potential of cationic liposomes (CLs) and magnetic cationic liposomes (MCLs) with two different concentrations of magnetite (MAG; 0.5 and 1 mg/mL) and their complexes with plasmid DNA (pDNA)
Figure 1 Gel retardation of (A) 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC)/cholesterol (Chol) at a molar ratio of 5:1 lipoplex; (B) DPPC/ dioctadecyldimethylammonium bromide (DOAB) at a molar ratio of 1:1 lipoplex; (C) DPPC/Chol/ DOAB at a molar ratio of 7:2:1 lipoplex; (D) magnetic cationic liposomes/pDNA with different concentrations of magnetite (MAG). pGL3 plasmid and empty liposomes were used as controls. For all panels, (1) is suspension in water, (2) is suspension in ethanol, and (3) is suspension in aqueous solution of plasmid.
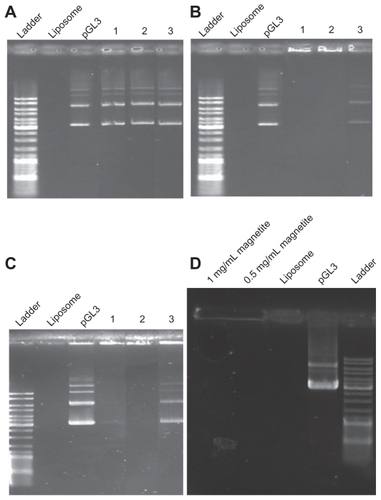
Figure 2 Comparing the transfection efficiencies of 1,2-dipalmitoyl-sn-glycero-3- phosphocholine (DPPC)/cholesterol (Chol)/dioctadecyldimethylammonium bromide (DOAB) and DPPC/DOAB liposomes with different molar ratios.
Abbreviation: RLU, relative light unit.
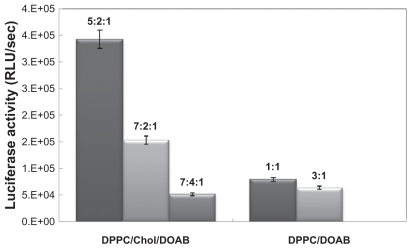
Figure 3 Comparison of luciferase assay in cells transfected by 1,2-dipalmitoyl-snglycero-3-phosphocholine (DPPC)/cholesterol (Chol)/dioctadecyldimethylammonium bromide (DOAB) liposomes composition at a molar ratio of 7:2:1 and DPPC/DOAB at a molar ratio of 1:1, and comparison of different methods of preparing them.
Abbreviation: RLU, relative light unit.
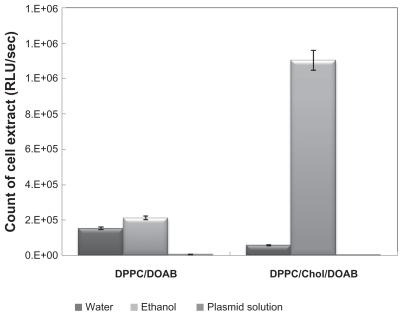
Figure 4 Optimizing the magnetic cationic liposome (MCL)/plasmid DNA (pDNA) N/P ratio evaluated by luciferase assay in Chinese hamster ovary cells.
Abbreviation: RLU, relative light unit.
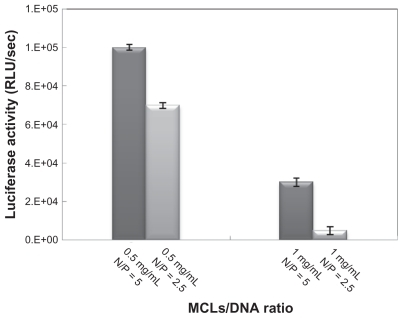
Figure 5 Optimization of incubation time evaluated by luciferase assay in Chinese hamster ovary cells with magnetic induction.
Abbreviations: MCL, magnetic cationic liposome; RLU, relative light unit.
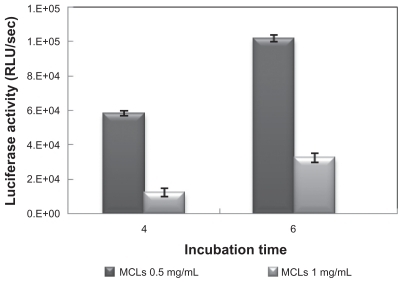
Figure 6 Comparing the nonmagnetic induction transfection efficiencies of lipoplexes under the optimal transfection conditions (6-hour incubation time) in Chinese hamster ovary cells.
Abbreviations: CL, cationic liposome; MCL, magnetic cationic liposome; RLU, relative light unit.
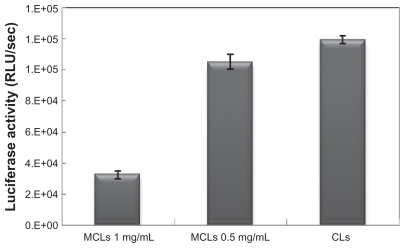
Figure 7 Effect of magnetic field exposure on transfection efficiency. (A) magnetic cationic liposomes (MCLs)/plasmid DNA (pDNA) lipoplex (MCLs with magnetite [MAG] 0.5 mg/mL concentration) after 6 hours’ incubation and (B) MCLs/pDNA lipoplex (MCLs with MAG 0.5 mg/mL concentration) after 4 hours’ incubation. These experiments were carried out under the optimal transfection conditions and magnetic induction time was varied from 30 to 60 minutes.
Abbreviation: RLU, relative light unit.
![Figure 7 Effect of magnetic field exposure on transfection efficiency. (A) magnetic cationic liposomes (MCLs)/plasmid DNA (pDNA) lipoplex (MCLs with magnetite [MAG] 0.5 mg/mL concentration) after 6 hours’ incubation and (B) MCLs/pDNA lipoplex (MCLs with MAG 0.5 mg/mL concentration) after 4 hours’ incubation. These experiments were carried out under the optimal transfection conditions and magnetic induction time was varied from 30 to 60 minutes.Abbreviation: RLU, relative light unit.](/cms/asset/2eabd5f6-95fe-409d-a8db-0a0190297a64/dijn_a_23074_f0007_b.jpg)
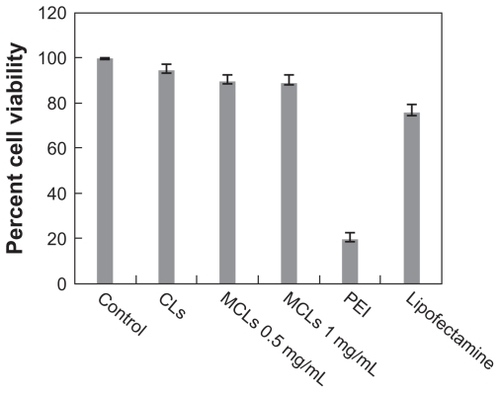
Figure 8 Viability of Chinese hamster ovary cells treated with cationic liposomes (CLs), magnetic cationic liposomes (MCLs), polyethyleneimine (PEI), and Lipofectamine™. Cells were seeded at 105 cells/mL in a 96-well plate and incubated at 37°C. Percentage of cell viability was determined following 24-hour exposure to varying amounts of MCLs (0.5 and 1 mg/mL of magnetite).
Note: Data represents the percentage of cell viability compared with untreated cells and cells treated with Lipofectamine and PEI as controls.