Figures & data
Table 1 Advantages and Disadvantages of Experimental Drugs Against High-Grade Gliomas
Figure 1 Main mechanisms of experimental drugs used against high-grade gliomas.
Abbreviations: avß3/avß5, avß3/avß5 heterodimers; EGFR, epidermal growth factor receptor; EGFRvIII, mutant EGFR; MMP, matrix metalloproteinase; PDGFR, platelet-derived growth factor receptor; PI3K/Akt/mTOR, phosphoinositide 3-kinase/protein kinase B/mammalian target of rapamycin; PKC, protein kinase C; PTEN, phosphatase and tensin homolog; Ras/MAPK, Ras mitogen-activated protein kinase; VEGF, vascular endothelial growth factor; VEGFR, vascular endothelial growth factor receptor.
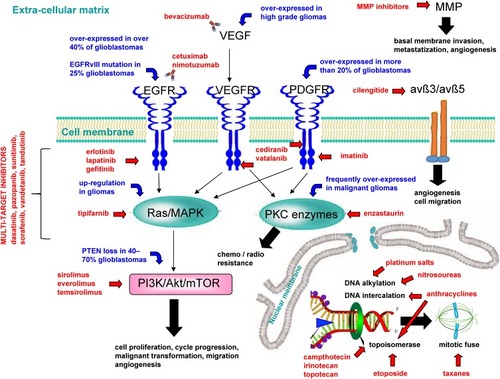
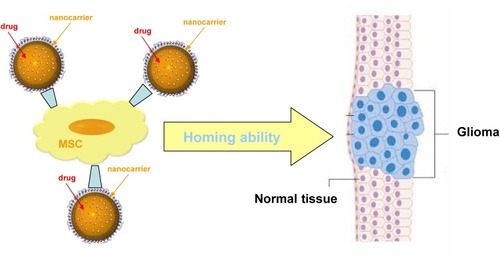
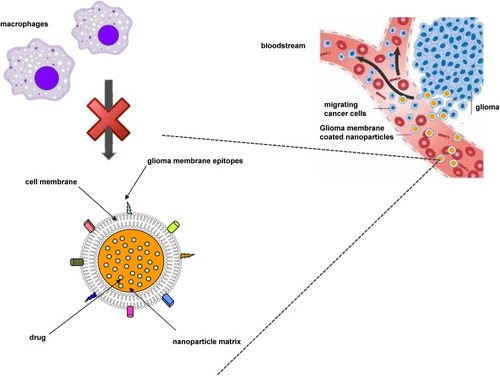
Figure 2 Rationale of employing nanomedicines for glioma treatment.
Abbreviations: BBB, blood–brain barrier; BBTB, blood–brain tumour barrier; CMT, carrier-mediated transport; EPR, enhanced permeation and retention; HIF, hypoxia-induced factors; P-gp, P-glycoprotein; RMT, receptor-mediated transport.
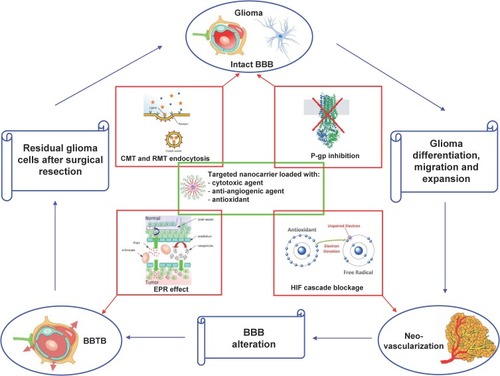
Figure 3 Scheme of brain delivery of nanoparticles with focused ultrasound (FUS) technique.
Abbreviation: SF6, sulfur hexafluoride.
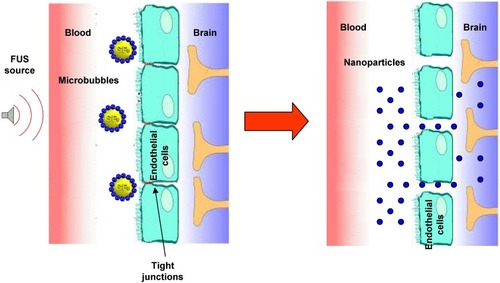
Table 2 Plain Nanocarriers Aiming to Glioma Therapy Employed in Preclinical Studies
Table 3 Functionalized Nanocarriers Aiming to Glioma Therapy Employed in Preclinical Studies
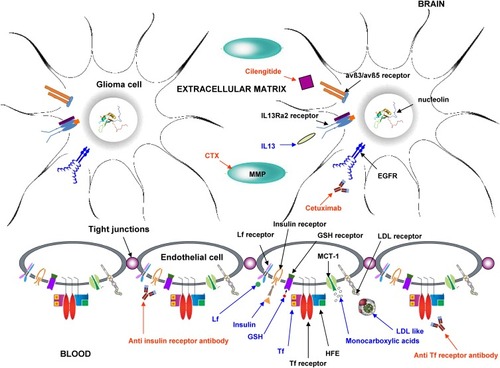
Figure 4 Scheme of the main mechanism used for active targeting of nanocarriers in glioma therapy. Blue: endogenous ligands; red: exogenous ligands.
Abbreviations: avß3/avß5, avß3/avß5 heterodimers; CTX, chlorotoxin; EGFR, epidermal growth factor receptor; GSH, glutathione; HFE, homeostatic iron regulator protein; IL 13, interleukin 13; LDL, low-density lipoprotein; Lf, lactoferrin; MCT-1, monocarboxylic acid transporter 1; MMP, matrix metalloproteinase; Tf, transferrin.