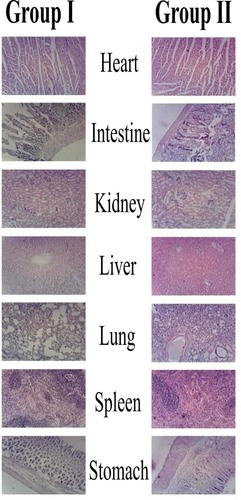
Figures & data
Table 1 Composition of Chitosan Loaded Nanoparticles Containing Docetaxel
Table 2 Evaluation of Different Formulation Parameters of Docetaxel Loaded Nanoparticles
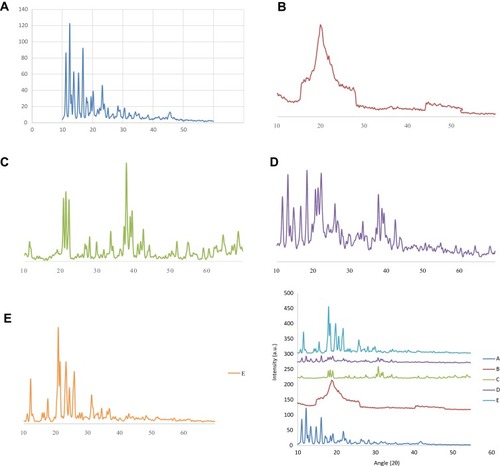
Figure 4 Comparative FTIR spectra of DTXL (A), chitosan (B), STPP (C), physical mixture (D) and formulation CNP3 (E).
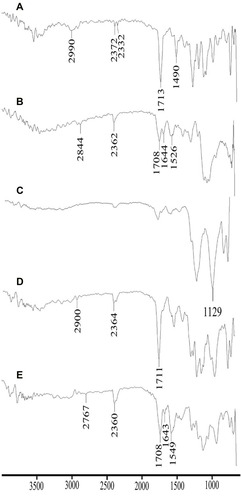
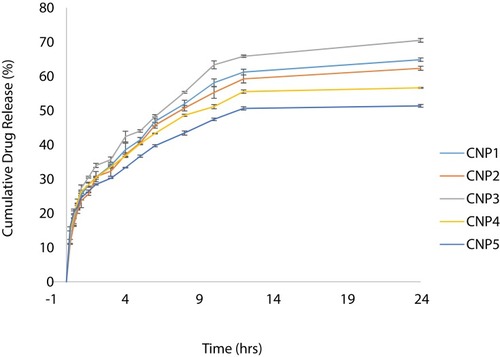
Table 3 Kinetic Modeling of Drug Release Profile of Chitosan Nanoparticles
Table 4 Clinical Observations of an Acute Oral Toxicity Study for Prepared Chitosan Loaded Nanoparticles
Table 5 Biochemical Blood Analysis
Table 6 Liver and Kidney Function Tests