Figures & data
Table 1 A Synopsis of the m Tuberculosis Main Virulence Factor and Their Pathogenic Mechanism
Table 2 A Synopsis on Studies Using AgNPs in the Treatment of Mycobacteria-Induced Diseases
Figure 1 Spatiotemporal dynamic model of the possible fates of Mycobacterium tuberculosis (MTb) following macrophage phagocytosis (1) MTb can prevent early phagosome maturation and by the action of Rab20-trafficking, the ESX-1 will destabilize and disrupt the phagosome membrane allowing MTb direct access into the macrophage cytosol, followed in certain conditions by MTb survival and multiplication; (2) Some early phagosomes will undergo normal maturation, will fuse with the lysosomes and MTb will be killed (by reactive nitrogen intermediates, low pH, ROS, antimicrobial peptides and Fe deprivation mediated by iron scavengers, as lactoferrin, and NRAMP1);Citation80 occasionally MTb can survive within the mature phagolysosome; (3) Blocking of the early phagosome maturation (mainly by inhibiting PI3P generation) followed by intravesicular MTb replication; (4) Delivery of the early endosomes or early-endosomes-to autolysosomes, where typically the activity of Mtb will be suppressed. Inspired from Philips et alCitation81 and Schnettger et al.Citation82 Figure 1 was created using BioRender.
Abbreviations: NRAMP1, natural resistance-associated macrophage protein 1.
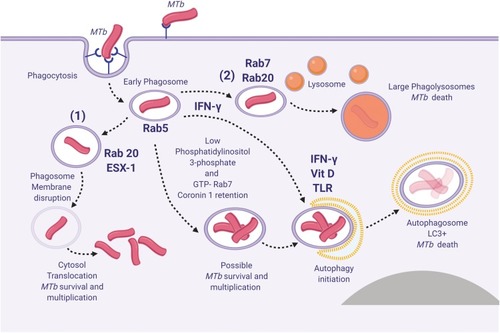
Figure 2 Histological characteristics of a tuberculous granuloma in the late caseo-calcareous stage. Image (A) “caseating tubercule” consisting of a large central area of caseating necrosis (zone 1) with extensive calcification (asterisk), surrounded by a reactive rim (zone 2) of lymphocytes and macrophages (including macrophage-derived epithelioid and multinucleated giant cells) and bordered by a partially formed fibrous capsule (zone 3) focally infiltrated by the above-mentioned cells; Image (B) detail of the leukocyte rim (zone 2), depicting several multinucleated giant cells (Langhans type) (arrowheads) admixed with fewer histiocytes, macrophages, and lymphocytes (arrow). Image (C and D) many acid-fast bacilli located intracellularly within the Langhans type multinucleate giant cells and histiocytes (image (C), arrowheads) and extracellularly (image (D), arrowheads). Image (A and B), Hematoxylin and eosin stain; Image (C and D) Ziehl–Neelsen stain for mycobacteria; ob x 4 for image (A) (scale bar=500 µm), x20 for image (B) (scale bar=100 µm), and x100 for images (C and D) (scale bar=20 µm).
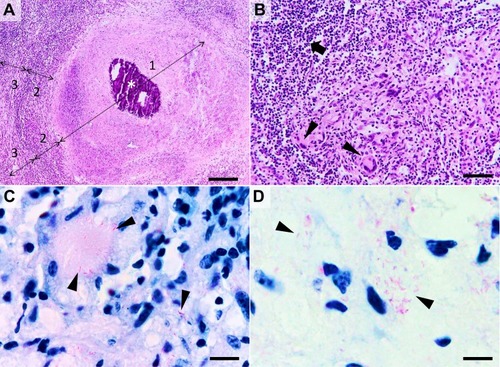
Figure 3 The three most important routes of antimicrobial action of AgNPs. 1. Accumulation and disruption of the extracellular polymers of the bacterial biofilm; silver ions (Ag+) could also biochemically alter the biofilm overall adherence, structure, and porosity.2. AgNPs adhere to bacterial cell surface (documented for Gram-positive, negative and also for the acid-fast bacteria) resulting in microbial membrane disruption, altered transmembranar transport, cellular content leakage (mainly electrolytes dysregulation) and bacterial death (apoptosis/lysis); as for the biofilm, Ag+ generated extracellularly contribute to the microbial cell wall disruption by biochemical alteration of the SH– groups. 3. AgNPs penetrate bacterial cell wall and access microbial cytoplasm where can interact with the organelles, cytosolic molecules (as free amino acids, peptides, and enzymes) and bacterial cytoskeleton; By direct action of AgNPs and Ag+ results the alteration of several metabolic pathways, bacterial organelles dysfunction (mainly mitochondria), ROS generation and bacterial DNA alteration ultimately causing cell death apoptosis/lysis). was created using BioRender.
Abbreviations: AgNPs, silver nanoparticle; ROS, reactive oxygen species: Ag+, silver ions.
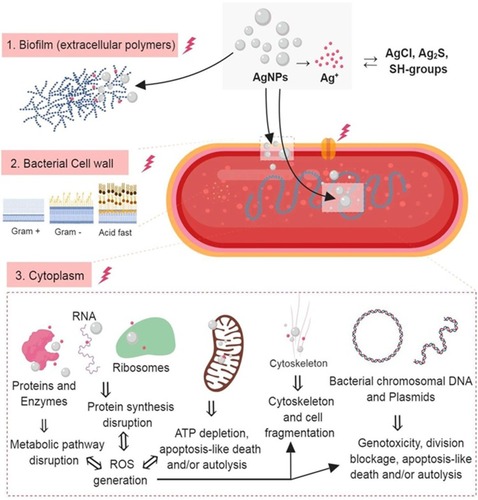
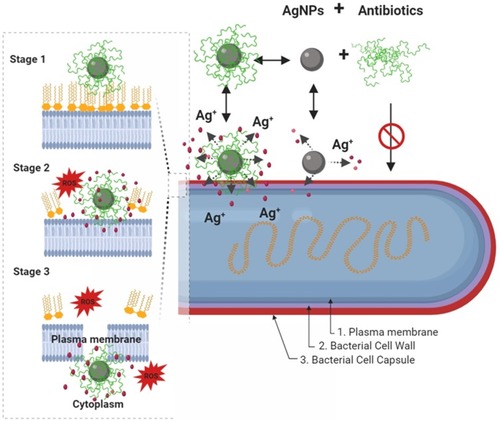
Figure 4 Schematic diagram showing, in a step by step fashion, the synergistic pathways and mechanisms of AgNP and antibiotics against multidrug-resistant bacteria (depicted in G- bacteria). Enhancement of the accumulation of the AgNPs conjugates with antibiotics within the bacterial cell membrane is associated with potentiation of Ag+ release and damage of the bacterial capsule, cell wall, and plasma membrane components. In this paradigm, the pathway mediated by AgNPs is a minor antibacterial mechanism, and the activity mediated by antibiotics-only is not effective due to antibacterial resistance. In a step by step diagram of the bacterial membrane destabilization (depicted for AgNPs/nisin conjugates), the interaction between AgNP/antibiotic complexes with bacterial cell membrane (stage 1) will results in enhancement Ag+ release, in situ ROS generation, membrane-insertion of nisin (methyl)-lanthionine rings, followed by local dissolution of lipidic molecules, membrane-pore formation, and internalization of AgNPs/nisin complexes within the bacterial cytoplasm. Inspired from Deng et alCitation198 and Arakha et alCitation206 schematic concepts of AgNP/nisin and AgNPs/tetracycline complexes-mediated antibacterial activity. Figure 4 was created using BioRender.
Abbreviations: AgNPs= silver nanoparticle; ROS, reactive oxy gen species: Ag+=silver ions.