Figures & data
Table 1 Different Properties Between Antibodies and Aptamers
Figure 1 Schematic illustration of the recognition process of S. aureus using a AuNCs@Van and Apt-MB (I) dual recognition assay, and the characterization (II) (A–F) represents the optical, fluorescence and transmission electron microscopy characterization of this synthesized particle. The figure was reprinted with permission from Cheng D, Yu M, Fu F, et al. Dual recognition strategy for specific and sensitive detection of bacteria using aptamer-coated magnetic beads and antibiotic-capped gold nanoclusters. Analytical Chemistry. 2016;88(1):820–825. Copyright © 2016 American Chemical Society.Citation43
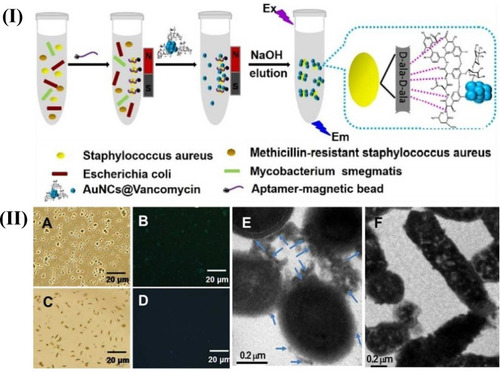
Table 2 Types of Aptamer-Based Nanostructures in Diagnostics with Their Advantages and Limitations
Figure 2 AuNPs-aptamer was capped on the MSA surface due to the binding reaction of ATP aptamer to the adenosine molecule. The delivery of the entrapped guest (fluorescein) was selectively triggered by an effective displacement reaction in the presence of the target molecule (ATP). Reprinted with permission from Zhu CL, Lu CH, Song XY, Yang HH, Wang XR. Bioresponsive controlled release using mesoporous silica nanoparticles capped with aptamer-based molecular gate. J Am Chem Soc. 2011;133(5):1278-1281. Copyright (2020) American Chemical Society.Citation73
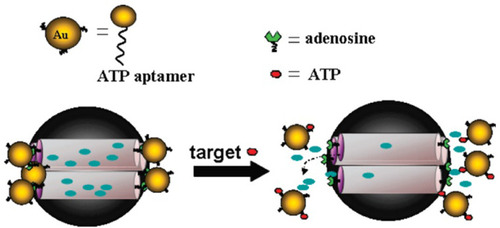
Figure 3 Schematic representation of cationic nanoparticles for targeted delivery of siRNA-aptamer chimeras. Immobilization of preformed siRNA-aptamer chimeras onto positively charged QD-PMAT-PEI nanoparticles. The aptamer block collapsed on the carrier results in reduced binding activity. Two-step immobilization of chimeras on a cationic nanoparticle surface. siRNA molecules with a thiol-reactive terminal group are first adsorbed on the QD-PMAT-PEI surface to reduce the positive charge; subsequently aptamers with a single thiol group are brought in to form siRNA-aptamer chimeras on the nanoparticle surface. Adapted with permission from Bagalkot V, Gao X. siRNA-aptamer chimeras on nanoparticles: preserving targeting functionality for effective gene silencing. ACS Nano. 2011;5(10):8131-8139.Citation82,Citation138 Copyright (2011) American Chemical Society.
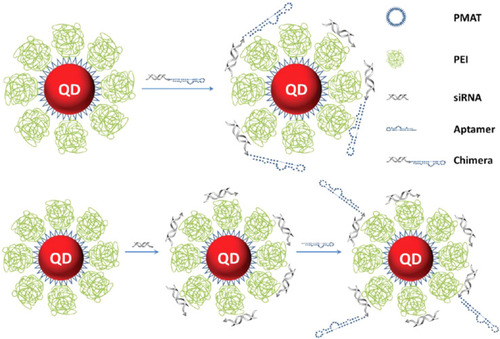
Figure 4 Schematic of the newly synthesized aptamer-PEI-siRNA nanocomplex (PEI-EpApt-SiEP) and its function to silence the target gene and decrease cell proliferation. The figure was reproduced from Subramanian N, Kanwar JR, Athalya P, et al. EpCAM aptamer mediated cancer cell specific delivery of EpCAM siRNA using polymeric nanocomplex. J Biomed Sci. 2015;22(1):4. Copyright © Subramanian et al; licensee BioMed Central. 2015. Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0).Citation98
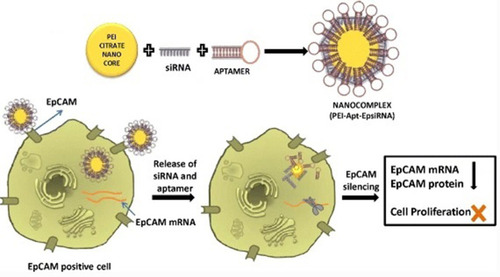
Figure 5 SEM images of carbon nanotube: DNA complexes formed at a 6:1 charge ratio: (A-C) MWNT-NH3 +:DNA; (D-F) SWNT-NH3 +:DNA. Reprinted with permission from Singh R, Pantarotto D, McCarthy D, et al. Binding and condensation of plasmid DNA onto functionalized carbon nanotubes: toward the construction of nanotube-based gene delivery vectors. J Am Chem Soc. 2005;127(12):4388-4396.Citation114,Citation139 Copyright (2020) American Chemical Society.

Table 3 Types of Aptamer-Based Structures in Gene Delivery Systems
