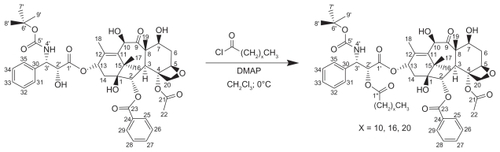
Figures & data
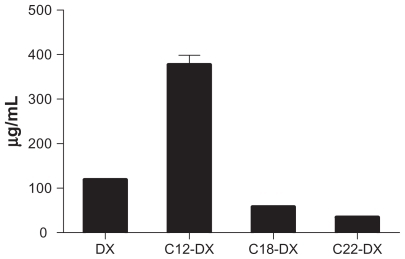
Table 1 Solubility of DX and DX conjugates in Miglyol 808 (N = 3)
Table 2 Orthogonal design and responses
Table 3 Compositions and properties of BTM 808 NPs
Figure 3 3D surface plot for the modeling of the effect of Brij 78 and TPGS concentrations on percent of entrapment.
Abbreviations: DX, docetaxel; TPGS, tocopheryl polyethylene glycol succinate.
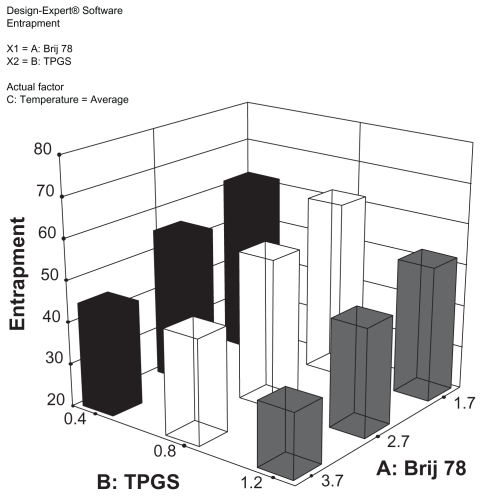
Figure 4 Release of DX conjugates from BTM NPs in mouse plasma.
Abbreviations: DX, docetaxel; BTM, Brij 78, Vitamin E TPGS and Miglyol 808.
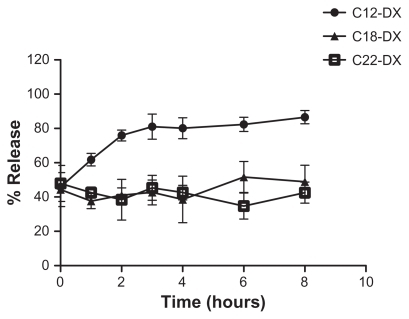
Figure 5 In vitro cytotoxicity of free DX and DX conjugates and their NPs in DU-145 cells.
Abbreviation: DX, docetaxel.
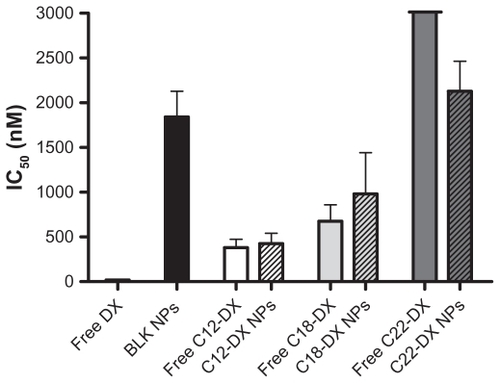
Figure 6 The digestion of free DX conjugates in fresh mouse plasma at 37°C.
Note: Data are shown as mean ± SD (n = 3).
Abbreviation: DX, docetaxel.
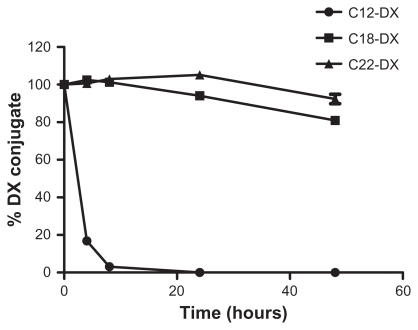
Table 4A Pharmacokinetic parameters of DX conjugates and Taxotere in mice after iv bolus administration
Figure 7 Plasma concentration-time curves for (A) DX, C12-DX, C18-DX, and C22-DX after administration of Taxotere, C12-DX NPs, C18-DX NPs, and C22-DX NPs, respectively, and (B) DX as an active metabolite from C12-DX NPs and C18-DX NPs using Taxotere as a reference. The plasma concentrations of DX from C22-DX NP were below the lower limit of quantification.
Note: Data are shown as mean ± SD (n = 3).
Abbreviation: DX, docetaxel.
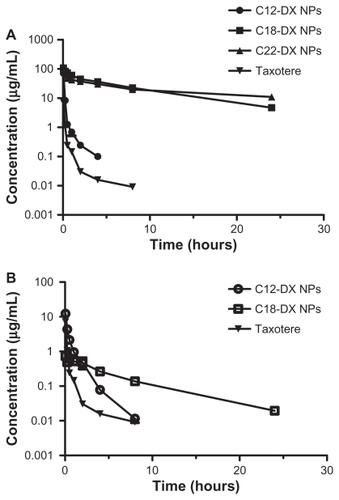
Table 4B Pharmacokinetic parameters of DX after iv bolus administration of DX conjugates and Taxotere in mice