Figures & data
Figure 1 Hierarchical self-assembly of rosette nanotubes. The G∧C motif functionalized with K and RGDSK self-assemble into six-membered supermacrocycles (rosettes), which then stack up to form rosette nanotubes. K–G∧C and RGDSK–G∧C can be co-assembled in a predefined molar ratio to form hybrid rosette nanotubes, such as the K90/RGDSKCitation10-RNT investigated in this report.
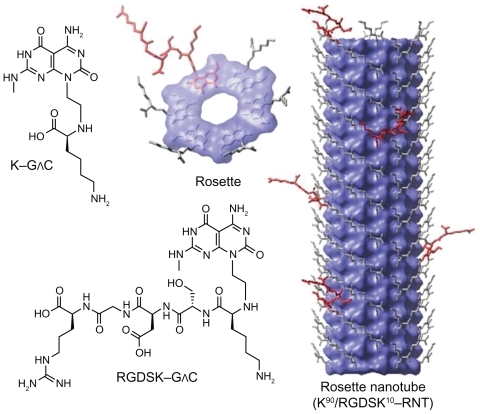
Figure 2 Histopathology of lung sections. Lungs from mice treated intranasally with 80 μg lipopolysaccharide and/or K90/RGDSKCitation10 rosette nanotubes (5%, 12.5 μL intravenously) and saline controls were evaluated using sections stained with hematoxylin and eosin. Control lung tissue showed no inflammation and normal lung architecture (A, B), whereas mice treated with K90/RGDSKCitation10 rosette nanotubes (C, D), lipopolysaccharide (E, F), and lipopolysaccharide + K90/RGDSKCitation10 rosette nanotubes (G, H) showed septal neutrophilic infiltration, leukocytes adhering to the blood vessel wall, perivascular infiltration of leukocytes, and peribronchiolar accumulation of neutrophils at the 12-hour time point.
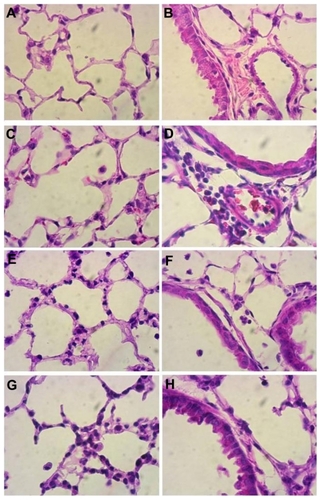
Figure 3 Differential cell count and protein in bronchoalveolar lavage fluid. Total and differential cell count was performed in bronchoalveolar lavage fluid 3, 6, or 12 hours following treatment. Numbers of polymorphonuclear cells in the bronchoalveolar lavage fluid were significantly higher in the lipopolysaccharide and K90/RGDSKCitation10 rosette nanotube groups compared with saline controls. However, the lipopolysaccharide + K90/RGDSKCitation10 rosette nanotube treatment group showed robust polymorphonuclear emigration at all time points (A). Lipopolysaccharide resulted in an increase in emigration of monocytes in bronchoalveolar lavage fluid after 6 hours (B). Treatment with K90/RGDSKCitation10 rosette nanotubes had no effect on monocyte emigration compared with the saline control at all time points tested. Interestingly, mice treated with lipopolysaccharide and K90/RGDSKCitation10 rosette nanotubes showed a significant increase in monocyte emigration. Total protein analysis in bronchoalveolar lavage fluid showed a significant increase in protein content in the lipopolysaccharide and K90/RGDSKCitation10 rosette nanotube treatment groups (C). Interestingly, K90/RGDSKCitation10 rosette nanotubes significantly proved the effect of lipopolysaccharide.
Note: Groups bearing different superscripts were significantly different (P < 0.05), while the groups with similar superscripts did not differ.
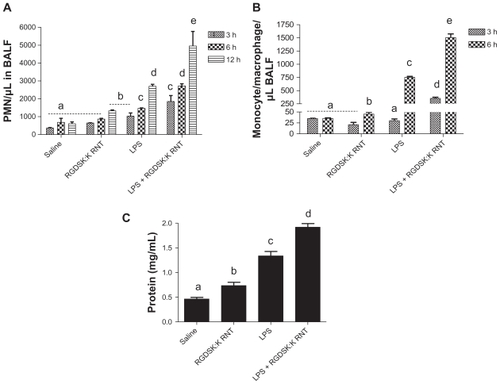
Figure 4 Myeloperoxidase assay in bronchoalveolar lavage fluid. A significant increase in myeloperoxidase was observed in all treatment groups compared with saline controls. Mice treated with K90/RGDSKCitation10 rosette nanotubes and lipopolysaccharide showed significant higher myeloperoxidase activity in bronchoalveolar lavage fluid than the lipopolysaccharide group.
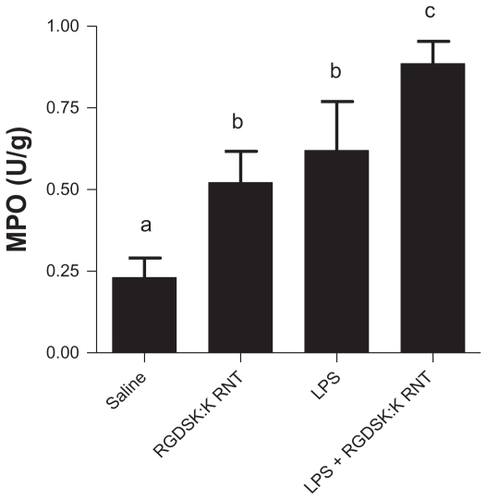
Figure 5 Secretion of proinflammatory cytokines. Lung homogenates from various treatment groups were analyzed for proinflammatory cytokines. Mice treated with K90/RGDSKCitation10 rosette nanotubes showed a significant increase in secretion of interleukin-1β, MIP-1, MCP-1, and KC-1 compared with the saline control and lipopolysaccharide groups. Interestingly, the K90/RGDSKCitation10 rosette nanotube + lipopolysaccharide group showed the highest levels of interleukin-1β, MIP-1, MCP-1, and KC-1.
Note: Groups bearing different superscripts were significantly different (P < 0.05), while the groups with similar superscripts did not differ.
Abbreviations: IL-1beta, interleukin-1beta; MCP-1, monocyte chemoattractant protein-1; KC-1, keratinocyte-dereived chemokine-1; MIP-1, macrophage inflammatory protein-1; ICAM, intercellular adhesion molecule.
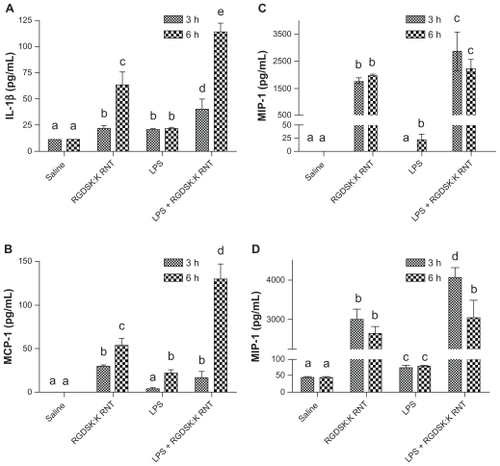
Figure 6 Expression of proinflammatory cytokines. The lipopolysaccharide and K90/RGDSKCitation10 rosette nanotube groups showed significantly higher expression of proinflammatory genes compared with saline controls. Mice treated with K90/RGDSKCitation10 rosette nanotubes following lipopolysaccharide challenge showed significant inhibition of interleukin-1β and MIP-1, and an increase in KC-1 mRNA transcripts when compared with the lipopolysaccharide group. MIP-1 expression did not differ between the lipopolysaccharide and lipopolysaccharide + K90/RGDSKCitation10 rosette nanotube groups.
Note: Groups bearing different superscripts were significantly different (P < 0.05), while the groups with similar superscripts did not differ.
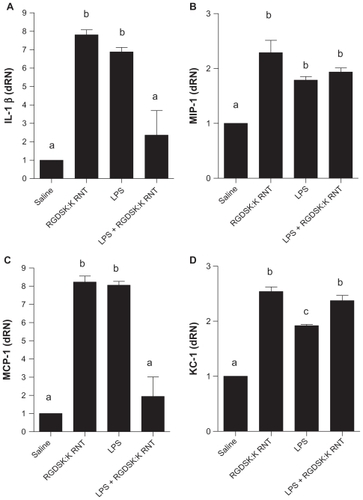
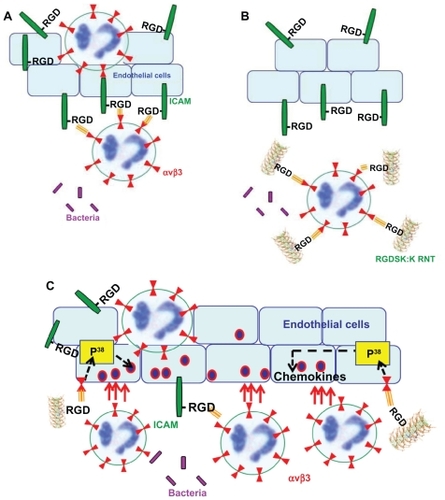
Figure 7 Possible mechanism of action of RGDSK:K rosette nanotubes in vivo. In acute lung inflammation, activated endothelial cells produce intercellular adhesion molecule-1 that contains a RGD motif, which is known to interact with leukocyte integrin αvβ3 resulting in the attachment of leukocytes to endothelial cells (A). Therefore, targeting neutrophil integrin αvβ3 with RGDSK (peptide or rosette nanotubes) might block free available receptors that may inhibit its transmigration across endothelial cells (B). Here we propose that K90/RGDSKCitation10 rosette nanotubes might be the triggering P38 mitogen-activated protein kinase cascade in vivo that upregulates the proinflammatory response (C).