Figures & data
Figure 1 Diagram showing mTORC1 and mTORC2 signaling pathways. Growth factors activate mTOR complex 1 (mTORC1) through IRS1/PI3K-PDK1-Akt by regulating the tuberous sclerosis complex (TSC)1/2. TSC functions as a GTPase activator protein (GAP) for the small G-protein Rheb, an upstream positive regulator of mTORC1. Amino acids signaling causes mTORC1 translocation to the lysosomes, where Rheb resides, via the Rag GTPases–Ragulator complex. S6K1-rpS6 and 4EBP1-eIF4E are well-known downstream targets of mTORC1 and are responsible for the translation pathway. mTORC1 also regulates lipid synthesis through SREBP and inhibits autophagy by phosphorylating TFEB and ULK1. mTORC2 controls cell metabolism, cell survival, and cytoskeleton rearrangement by activating Akt, SGK1, and PKC. Akt activity is regulated by both PDK1 and mTORC2. Dotted lines indicate feedback mechanisms.
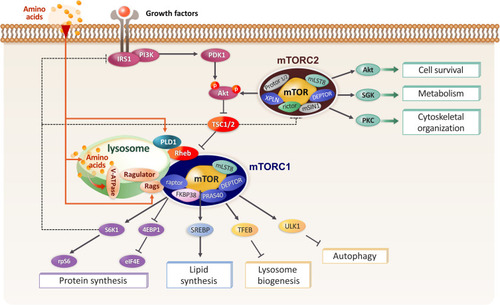
Figure 2 Diagram showing the components of mTORC1 upstream signaling on the lysosome mTORC1 is regulated by amino acid sensors and several multiprotein complexes which regulate Rag GTPases. LeuRS/Vps34/PLD1 also activates mTORC1 through PA during amino acid stimulation (see “mTORC1 Activation on the Lysosome” section).
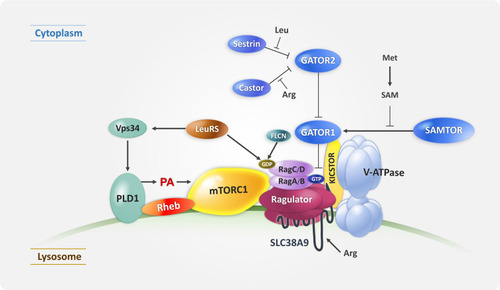
Figure 3 Diagram showing the mTOR structural domain targets for three generations of mTOR inhibitors. mTOR consists of several structural domains: HEAT repeats, FAT (for FRAP, ATM, TRAP), FRB (FK506 binding protein 12 (FKBP12)–rapamycin binding), kinase, and FATC (for C-terminal FAT) domains. As the name implies, the FRB domain is responsible for the binding of mTOR to FKBP12 and rapamycin. FAT, kinase, and FATC domains are required for maintaining phosphatidylinositol 3-kinase-related kinases (PIKKs) activity. Rapalogs, the first-generation mTOR inhibitors, decrease mTOR activity by interacting with the FRB domain of mTOR. The second-generation mTOR inhibitors competes with ATP for binding to the kinase domain of mTOR. RapaLink, the third-generation mTOR inhibitor, was developed to overcome the limitations of previous mTOR inhibitors. FRB domain mutations (mTORA2034V and mTORF2108L) and a kinase domain mutation (mTORM2327I) contribute to the resistance of mTOR to rapalogs and mTOR kinase inhibitors, respectively.
Table 1 Summary of Applications to Prevent Nanoparticle Uptake by Mononuclear Phagocyte System (MPS)
Table 2 Modulation of mTOR Signaling by Nanoparticles
