Figures & data
Figure 1 Hemostatic gauze scaffold implants. (A) Implant, (B) release on day 1, (C) release on day 2, and (D) release on day 7.
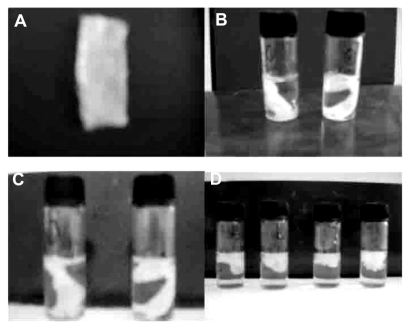
Figure 2 Scanning electron micrographs of different samples (A) G-CSF dextran nanoparticles, (B) G-CSF dextran nanoparticles from hemostatic gauze scaffold containing G-CSF-loaded dextran nanoparticles, (C) blank hemostatic gauze scaffold, (D) hemostatic gauze scaffold containing G-CSF-loaded dextran nanoparticles, and (E) cross-section from hemostatic gauze scaffold containing G-CSF-loaded dextran nanoparticles. (F) release on day 1, (G) release on day 2, and (H) release on day 7.
Abbreviation: G-CSF, granulocyte-colony stimulating factor.
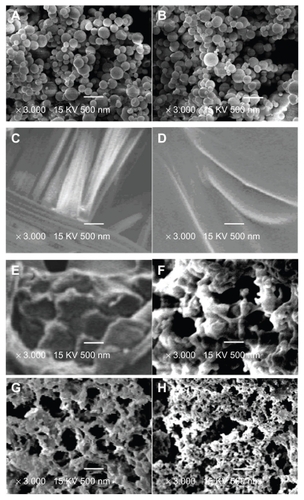
Figure 3 Monomer content of recovery G-CSF from the scaffold by size exclusion chromatography-high pressure liquid chromatography (n = 5, *P > 0.05, **P < 0.05). (A) G-CSF (G-CSF:dextran = 1.4:5.0 ± 0.3 mg) solution; (B) G-CSF from G-CSF-loaded dextran nanoparticles; (C) G-CSF from hemostatic gauze scaffold containing G-CSF-loaded dextran nanoparticles (PLGA 50/50 3A: first coating = 20.0 ± 0.5 mg PLGA, G-CSF-loaded dextran nanoparticles (G-CSF:dextran, 1:4) = 5.0 ± 0.3 mg, second coating (blank) = 14.0 ± 0.6 mg PLGA); (D) G-CSF from hemostatic gauze scaffold containing G-CSF-loaded dextran nanoparticles (PLGA 50/50 3A: first coating = 20.0 ± 0.5 mg PLGA, G-CSF solution [G-CSF:dextran = 1:4] = 5.0 ± 0.3 mg, second coating [blank] = 14.0 ± 0.6 mg PLGA).
Abbreviations: G-CSF, granulocyte-colony stimulating factor; PLGA, polylacticco- glycolic acid.
![Figure 3 Monomer content of recovery G-CSF from the scaffold by size exclusion chromatography-high pressure liquid chromatography (n = 5, *P > 0.05, **P < 0.05). (A) G-CSF (G-CSF:dextran = 1.4:5.0 ± 0.3 mg) solution; (B) G-CSF from G-CSF-loaded dextran nanoparticles; (C) G-CSF from hemostatic gauze scaffold containing G-CSF-loaded dextran nanoparticles (PLGA 50/50 3A: first coating = 20.0 ± 0.5 mg PLGA, G-CSF-loaded dextran nanoparticles (G-CSF:dextran, 1:4) = 5.0 ± 0.3 mg, second coating (blank) = 14.0 ± 0.6 mg PLGA); (D) G-CSF from hemostatic gauze scaffold containing G-CSF-loaded dextran nanoparticles (PLGA 50/50 3A: first coating = 20.0 ± 0.5 mg PLGA, G-CSF solution [G-CSF:dextran = 1:4] = 5.0 ± 0.3 mg, second coating [blank] = 14.0 ± 0.6 mg PLGA).Abbreviations: G-CSF, granulocyte-colony stimulating factor; PLGA, polylacticco- glycolic acid.](/cms/asset/2f95c202-d7b5-4dd0-9a2a-a7b8128a2e5b/dijn_a_26006_f0003_b.jpg)
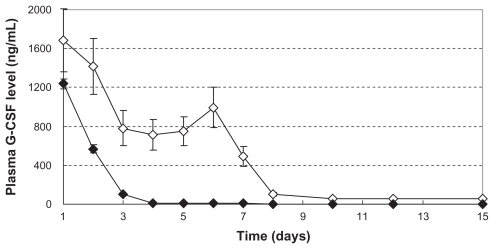
Figure 4 Recovery bioactivity from a hemostatic gauze scaffold containing G-CSF-loaded dextran nanoparticles (n = 5, *P > 0.05, **P < 0.05). Samples (A), (B), (C), and (E) were the same as for , respectively (n = 5, *P > 0.05, **P < 0.05). (D) Hemostatic gauze scaffold containing G-CSF from G-CSF-loaded dextran nanoparticles (PLGA 50/50 1A: first coating = 20.0 ± 0.6 mg PLGA, G-CSF loaded-dextran nanoparticles [G-CSF: dextran = 1:4] = 5.0 ± 0.2 mg, second coating [blank] = 14.0 ± 0.5 mg PLGA), (F) G-CSF from hemostatic gauze scaffold containing G-CSF-loaded dextran nanoparticles (PLGA 50/50 1A: first coating = 20.0 ± 0.6 mg PLGA, G-CSF solution [G-CSF:dextran = 1:4] = 5.0 ± 0.2 mg, second coating [blank] = 14.0 ± 0.5 mg PLGA).
Abbreviations: G-CSF, granulocyte-colony stimulating factor; PLGA, polylactic-co-glycolic acid.
![Figure 4 Recovery bioactivity from a hemostatic gauze scaffold containing G-CSF-loaded dextran nanoparticles (n = 5, *P > 0.05, **P < 0.05). Samples (A), (B), (C), and (E) were the same as for Figure 3A, B, C, and D, respectively (n = 5, *P > 0.05, **P < 0.05). (D) Hemostatic gauze scaffold containing G-CSF from G-CSF-loaded dextran nanoparticles (PLGA 50/50 1A: first coating = 20.0 ± 0.6 mg PLGA, G-CSF loaded-dextran nanoparticles [G-CSF: dextran = 1:4] = 5.0 ± 0.2 mg, second coating [blank] = 14.0 ± 0.5 mg PLGA), (F) G-CSF from hemostatic gauze scaffold containing G-CSF-loaded dextran nanoparticles (PLGA 50/50 1A: first coating = 20.0 ± 0.6 mg PLGA, G-CSF solution [G-CSF:dextran = 1:4] = 5.0 ± 0.2 mg, second coating [blank] = 14.0 ± 0.5 mg PLGA).Abbreviations: G-CSF, granulocyte-colony stimulating factor; PLGA, polylactic-co-glycolic acid.](/cms/asset/1e5f7198-5c27-4c68-8994-0f8a4dca1e2a/dijn_a_26006_f0004_b.jpg)
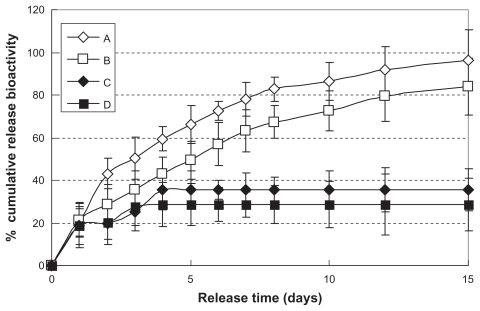
Figure 5 In vitro release profiles of hemostatic gauze scaffold containing G-CSF-loaded dextran nanoparticles (n = 5, P < 0.05). Samples (A), (B), (C), and (D) were the same as for (C), (D), (E), and (F), respectively.
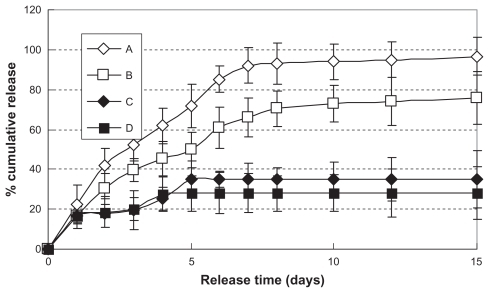
Figure 6 In vitro release relative bioactivity of hemostatic gauze scaffold containing G-CSF-loaded dextran nanoparticles (n = 5, P < 0.05). Samples (A), (B), (C), and (D) were the same as for (C), (D), (E), and (F), respectively.
Abbreviation: G-CSF, granulocyte-colony stimulating factor.