Figures & data
Table 1 Distribution of different NaFl formulations in the stratum corneum and viable skin
Figure 1 Characteristics of the ovalbumin (OVA)-loaded liposome 1 and 2. (A) Particle sizes and zeta potentials; (B) size distribution of the liposomes as determined by Malvern Zetamaster ([i]: liposome 1; [ii] liposome 2). (C) Vesicle morphology of the liposomes under transmission electron microscopy ([i]: liposome 1; [ii] liposome 2). (D) Sodium dodecyl sulfate polyacrylamide gel electrophoresis gel displaying the OVA encapsulated in liposome 1 and liposome 2 after loading process. Lane I: OVA standard; lane II: OVA loaded in liposome 1; lane III: OVA loaded in liposome 2.
![Figure 1 Characteristics of the ovalbumin (OVA)-loaded liposome 1 and 2. (A) Particle sizes and zeta potentials; (B) size distribution of the liposomes as determined by Malvern Zetamaster ([i]: liposome 1; [ii] liposome 2). (C) Vesicle morphology of the liposomes under transmission electron microscopy ([i]: liposome 1; [ii] liposome 2). (D) Sodium dodecyl sulfate polyacrylamide gel electrophoresis gel displaying the OVA encapsulated in liposome 1 and liposome 2 after loading process. Lane I: OVA standard; lane II: OVA loaded in liposome 1; lane III: OVA loaded in liposome 2.](/cms/asset/6df81f32-df3e-416e-ac50-a8ac1f42c53d/dijn_a_26152_f0001_c.jpg)
Figure 2 In vitro ovalbumin (OVA) release from the liposomes into phosphate-buffered saline (37°C). Liposome 1: prepared by film-dispersion method; liposome 2: prepared by reverse-evaporation method.
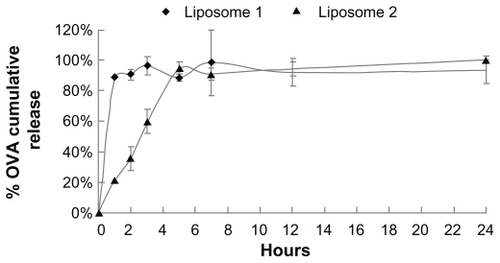
Figure 3 Fluorescent scanning microscopy images of skin samples from mice in which fluorescent ovalbumin (OVA) flexible liposomes had been applied transcutaneously: (Ai–Ci and Aiii–Ciii) images under visible light; (Aii–Cii and Aiv–Civ) images under fluorescence. Group A: OVA-loaded liposomes (blank control); Group B: FITC-OVA-loaded liposome by reverse-evaporation method; Group C: FITC-OVA-loaded liposome by film-dispersion method.
Notes: (Ai–Cii) are shown at ×5 magnification; (Aiii–Civ) are shown at ×20 magnification.
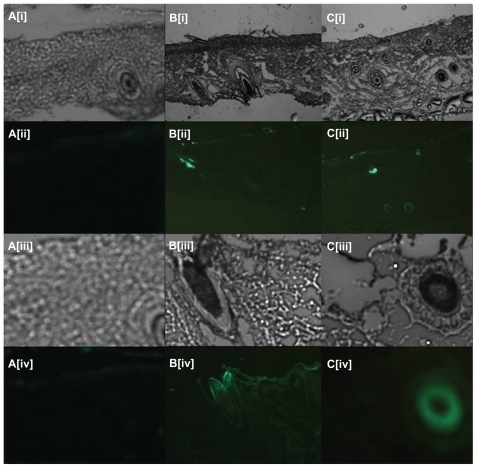
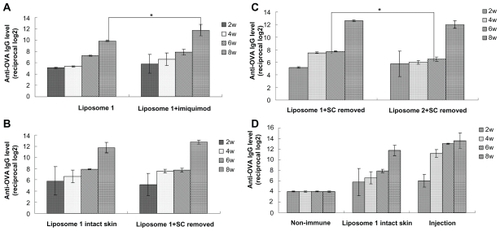
Figure 4 Anti-OVA IgG responses obtained with liposome formulations by transcutaneous immunization. (A) Liposome 1 (prepared by film-dispersion method) with or without adjuvant imiquimod transcutaneously. (B) Liposome 1 (prepared by film-dispersion method) applied on intact skin or stratum corneum pretreated skin transcutaneously, imiquimod as an adjuvant. (C) liposome 1 (prepared by film-dispersion method) and liposome 2 applied transcutaneously stratum corneum pretreated skin with the adjuvant imiquimod. (D) Liposome 1 (prepared by film-dispersion method) applied transcutaneously in the intact skin, with the adjuvant imiquimod; injection represents the OVA solution subcutaneously injected as a positive control.
Notes: Bars indicate standard deviation (n = 3). Statistical significance was evaluated by one-way ANOVA (*P < 0.05).
Abbreviations: OVA, ovalbumin; IgG, immunoglobulin G; ANOVA, analysis of variance.