Figures & data
Figure 1 (A) Time course of carboplatin loading by passive equilibration method into different liposomes; (B) representative cryogenic transmission electron microscopy images of empty and carboplatin-loaded liposomes* from at least three liposome batches.
Notes: Results shown are the mean plus or minus the standard error of the mean obtained from at least three independent experiments; ♦ = DPPC/DSPE-PEG1000 [molar ratio, 95:5], or DPPC-NT); *■ = DPPC/DSPE-PEG1000/DSPE-PEG2000-folate [molar ratio, 95:4.8:0.2], or DPPC-FRT); ▴ = DSPC/DSPE-PEG1000 [molar ratio, 95:5], or DSPC-NT); ● = DSPC/DSPE-PEG1000/DSPE-PEG2000-folate (molar ratio, 95:4.8:0.2; DSPC-FRT).
![Figure 1 (A) Time course of carboplatin loading by passive equilibration method into different liposomes; (B) representative cryogenic transmission electron microscopy images of empty and carboplatin-loaded liposomes* from at least three liposome batches.Notes: Results shown are the mean plus or minus the standard error of the mean obtained from at least three independent experiments; ♦ = DPPC/DSPE-PEG1000 [molar ratio, 95:5], or DPPC-NT); *■ = DPPC/DSPE-PEG1000/DSPE-PEG2000-folate [molar ratio, 95:4.8:0.2], or DPPC-FRT); ▴ = DSPC/DSPE-PEG1000 [molar ratio, 95:5], or DSPC-NT); ● = DSPC/DSPE-PEG1000/DSPE-PEG2000-folate (molar ratio, 95:4.8:0.2; DSPC-FRT).](/cms/asset/4ddfa283-55c3-46cf-9024-a641b2d6c86c/dijn_a_26172_f0001_b.jpg)
Figure 2 Cumulative release of carboplatin from various liposomes at 37°C under sink conditions (1:1000 v/v) in (A) phosphate-buffered saline and (B) 50% (v/v) fetal bovine serum.
Notes: Results shown are the mean plus or minus the standard error of the mean obtained from at least three independent experiments; ♦ = DPPC/DSPE-PEG1000 [molar ratio, 95:5], or DPPC-NT); ■ = DPPC/DSPE-PEG1000/DSPE-PEG2000-folate [molar ratio, 95:4.8:0.2], or DPPC-FRT); ▴ = DSPC/DSPE-PEG1000 [molar ratio, 95:5], or DSPC-NT); ● = DSPC/DSPE-PEG1000/DSPE-PEG2000-folate (molar ratio, 95:4.8:0.2; DSPC-FRT).
![Figure 2 Cumulative release of carboplatin from various liposomes at 37°C under sink conditions (1:1000 v/v) in (A) phosphate-buffered saline and (B) 50% (v/v) fetal bovine serum.Notes: Results shown are the mean plus or minus the standard error of the mean obtained from at least three independent experiments; ♦ = DPPC/DSPE-PEG1000 [molar ratio, 95:5], or DPPC-NT); ■ = DPPC/DSPE-PEG1000/DSPE-PEG2000-folate [molar ratio, 95:4.8:0.2], or DPPC-FRT); ▴ = DSPC/DSPE-PEG1000 [molar ratio, 95:5], or DSPC-NT); ● = DSPC/DSPE-PEG1000/DSPE-PEG2000-folate (molar ratio, 95:4.8:0.2; DSPC-FRT).](/cms/asset/e423d6f6-70b3-4812-a016-cb5d512c5578/dijn_a_26172_f0002_b.jpg)
Figure 3 (A) Cellular accumulation of DiI-labeled folate receptor-targeted (FRT) liposomes (i), nontargeted (NT) liposomes (ii), and FRT liposomes in the presence of 1 mM of free folic acid (iii) in IGROV-1 cells after 24 hours. (B) Cellular platinum (Pt) content in IGROV-1 cells after treatment with free carboplatin, carboplatin-loaded NT liposomes, or carboplatin-loaded FRT liposomes.
Notes: Images are representative of three independent experiments; data represent the mean plus or minus the standard error of the mean obtained from three independent experiments.
Abbreviation: ppb, parts per billion.
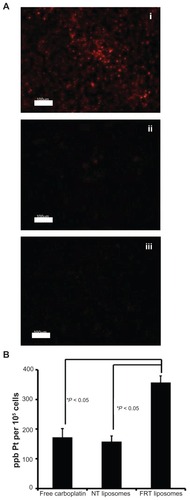
Table 1 IC50 values of free carboplatin, carboplatin-loaded nontargeted (NT) liposomes, and carboplatin-loaded folate receptor-targeted (FRT) carboplatin liposomes in IGROV-1 cells
Figure 4 Viability of IGROV-1 cells upon 72-hour exposure to free carboplatin (), carboplatin-loaded nontargeted liposome (♦), carboplatin-loaded folate receptor-targeted liposome (■), and carboplatin-loaded folate receptor-targeted liposome with 1 mM folic acid (×).
Note: Data represent the mean plus or minus the standard error of the mean obtained from five independent experiments.
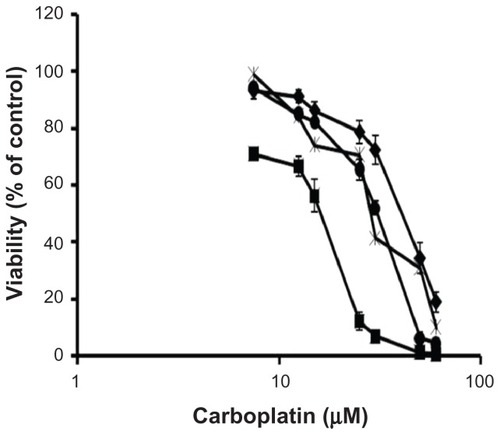
Table 2 In vivo efficacy of intraperitoneal administration of carboplatin in various forms in intraperitoneal IGROV-1 ovarian tumor xenograft
Figure 5 (A) Kaplan–Meier survival curves of mice bearing intraperitoneal IGROV-1 ovarian tumor xenograft treated with saline (blue), free carboplatin (green), carboplatin-loaded nontargeted (NT) liposomes (red) and carboplatin-loaded folate receptor-targeted (FRT) liposomes (black). Each study group comprised six animals. (B) Plasma drug concentrations (conc) in animals treated with various intraperitoneal carboplatin formulations at a dose of 15 mg/kg. Carboplatin was administered for a total of six doses, and plasma samples were collected 24 hours after doses 2, 4, and 6.
Notes: Results shown in panel (B) were obtained from at least three mice. The exception to this was dose 6, with n = 2 and n = 1 for free drug and carboplatin-loaded NT liposome groups, respectively.
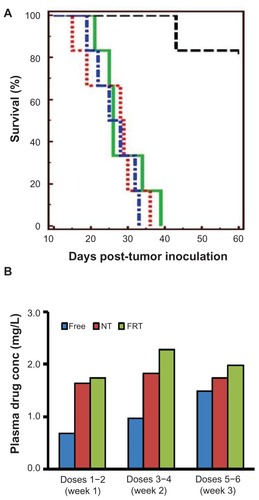
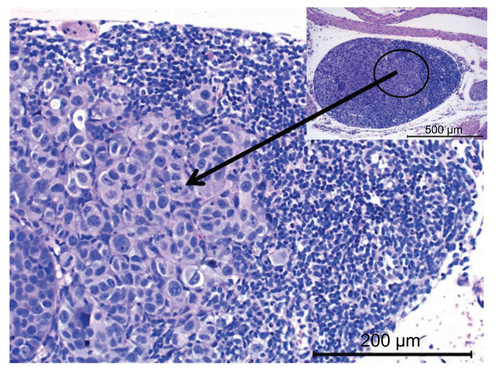
Figure 6 Histopathological evaluation of excised lung tissue (top) and liver tissue (bottom) indicating the presence of cancer cells in mice treated with (A) saline, (B) free carboplatin, and (C) carboplatin-loaded nontargeted liposome. Mice treated with carboplatin-loaded folate receptor-targeted liposome (D) that survived the study period did not show any presence of cancer cells.
Notes: Representative images are shown from at least three animals, with arrows indicating the presence of cancer cells.
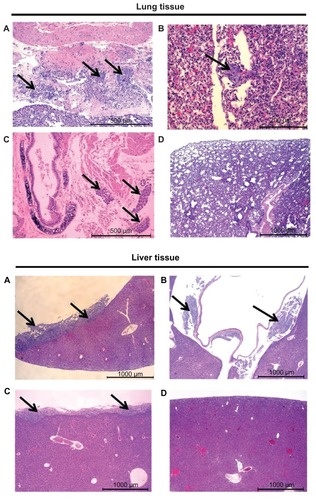
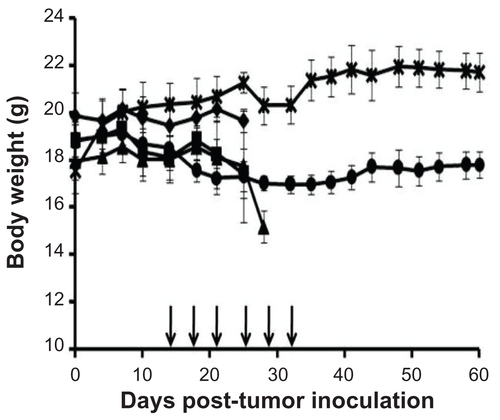
Figure S1 Changes in body weight in various study groups, including saline (◆), free carboplatin (■), carboplatin-loaded nontargeted liposome (▴), carboplatin-loaded folate receptor-targeted liposme (●), and healthy control mice (×).
Notes: Body weights were measured at least three times a week, with the arrows indicating the intraperitoneal administration of treatment. Results shown are the mean plus or minus the standard error of the mean obtained from at least three mice.